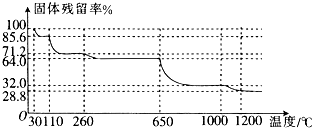
��Ŀ����
9��A��B��C��D����ԭ������������������ֶ����ڷǽ���Ԫ�أ���������������������C��ԭ��������A��B��ԭ������֮�ͣ�A��C��D��������������Ϊ13��
��D��ԭ��������C��2����D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣮ
�Ը������������ش�
��1��B���ʵĵ���ʽΪ
 ������CԪ�ص�ԭ�ӽṹʾ��ͼ
������CԪ�ص�ԭ�ӽṹʾ��ͼ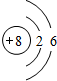 ��
����2�����л���������B��C�γɵĻ������йص���BC��
A������ЧӦ B���⻯ѧ���� C������ D��PM2.5��
���� A��B��C��D����ԭ������������������ֶ�����Ԫ�أ�D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣬ��DΪ��Ԫ�أ�D��ԭ��������C��2������C��ԭ������Ϊ8����CΪ��Ԫ�أ�A��C��D��������������Ϊ13����A������������Ϊ13-6-6=1��A���ڢ�A�壬��AΪH����B��ԭ������Ϊ8-1=7����BΪ��Ԫ�أ���AΪLi����B��ԭ������Ϊ8-3=5����BΪ��Ԫ�أ�����⣨2����֪��AΪ��Ԫ�ء�BΪ��Ԫ�أ��ݴ˽��
��� �⣺A��B��C��D����ԭ������������������ֶ�����Ԫ�أ�D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣬ��DΪ��Ԫ�أ�D��ԭ��������C��2������C��ԭ������Ϊ8����CΪ��Ԫ�أ�A��C��D��������������Ϊ13����A������������Ϊ13-6-6=1��A���ڢ�A�壬��AΪH����B��ԭ������Ϊ8-1=7����BΪ��Ԫ�أ���AΪLi����B��ԭ������Ϊ8-3=5����BΪ��Ԫ�أ�����⣨2����֪��AΪ��Ԫ�ء�BΪ��Ԫ�أ�
��1��B����Ϊ�����������е�ԭ��֮���γ�3�Թ��õ��Ӷԣ��ʵ���ʽΪΪ ��OԪ��������Ϊ8�����������Ϊ8����2�����Ӳ㣬����������Ϊ6������ԭ�ӽṹʾ��ͼ
��OԪ��������Ϊ8�����������Ϊ8����2�����Ӳ㣬����������Ϊ6������ԭ�ӽṹʾ��ͼ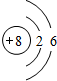 ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2����Ԫ�ص���������Ե��¹⻯ѧ���������꣬PM2.5���ɷ۳����¡�����ЧӦ�ɶ�����̼�ȵ��£�
�ʴ�Ϊ��BC��
���� ���⿼��ṹ����λ�ù�ϵ�����û�ѧ���������Ⱦ�ȣ�ע�����֪ʶ���������գ�

| A�� | �γ�̼�� | B�� | �γ�̼�� | C�� | ͨ�����Ӽ� | D�� | ͨ�����ۼ� |
| A�� | �����£���Ũ�ȵ��Ȼ�狀ͷ������Һ�������Ũ��ǰ�ߴ� | |
| B�� | CH3COOH��Һ��ˮϡ�ͺ���Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}CO{O}^{-}��}$��ֵ��С | |
| C�� | �����£�pH��ȵĴ����ƺ�̼��������Һǰ�ߵ����ʵ���Ũ�Ƚϴ� | |
| D�� | �����£�NaClO��Һ�У�c��HClO��+c��ClO-��=c��Na+�� |
| A�� | ����ȼ�ϵ�����ڻ����Ѻõ�� | |
| B�� | ͨO2�ļ���������ͨ��H2�ļ��Ǹ��� | |
| C�� | ����һ��ʱ���KOH��Һ������������������ | |
| D�� | ����ʱ��Һ�е�OH-���������ƶ� |
| A�� | .�� | B�� | �� | C�� | .�� | D�� | .�� |
| A�� | �����淴Ӧ���ʶ������� | B�� | HI��H2��I2��Ũ����� | ||
| C�� | HI��H2��I2�������й��� | D�� | HI��H2��I2��Ũ�Ⱦ����ٱ仯 |
H2��g��+$\frac{1}{2}$O2 ��g���TH2O��l����H1
C��s��+O2��g���TCO2��g����H2
CH3COOH��l��+2O2��g���T2CO2��g��+2H2O��l����H3
2C��s��+2H2��g��+O2��g���TCH3COOH��l����H4
���H4����ȷ����ʽΪ��������
| A�� | ��H3-2��H1-2��H2 | B�� | 2��H1+2��H2-��H3 | C�� | 2��H1-2��H2+��H3 | D�� | 2��H1-2��H2-��H3 |