��Ŀ����
7����Ҫ��д�Ȼ�ѧ����ʽ����1����֪ϡ��Һ�У�1mol H2SO4��NaOH��Һǡ����ȫ��Ӧʱ���ų�114.6kJ������д����ʾH2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽNaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2��25�桢101kPa�����³��ȼ��1kg�������ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ5��104 kJ��д����ʾ����ȼ���Ȼ�ѧ����ʽC4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��H=-2900kJ/mol��
��3����֪�����Ȼ�ѧ����ʽ��
��CH3COOH��l��+2O2��g���T�T2CO2��g��+2H2O��l����H1=-870.3kJ/mol
��C��s��+O2��g���T�TCO2��g����H2=-393.5kJ/mol
��H2��g��+$\frac{1}{2}$O2��g���T�TH2O��l ����H3=-285.8kJ/mol
д����C��s����H2��g����O2��g����������CH3COOH��l�����Ȼ�ѧ����ʽ2C��s��+2H2��g��+O2��g���TCH3COOH��l����H=-488.3kJ/mol��
��4����֪��C��s��ʯī��+O2��g��=CO2��g����H1=Q1kJ•mol-1
2CO2��g��+H2 ��g��=C2H2��g��+2O2��g����H2=Q2kJ•mol-1��
д����C��s��ʯī����H2��g������C2H2��g�����Ȼ�ѧ����ʽ��2C��ʯī��s��+H2��g���TC2H2��g����H=��2Q1+Q2��kJ/mol��
���� ��1�������к��ȵĸ��ϡ��ǿ���ǿ�Ӧ����1molˮ���ų�����������к����Լ��к��ȵ��Ȼ�ѧ����ʽ��
��2������ȼ���ȵĸ�����Ȼ�ѧ����ʽ����д����д����ע�����ʵľۼ�״̬���ʱ�ļ��㣻
��3����֪����CH3COOH��l��+2O2��g���T2CO2��g��+2H2O��l����H1=-870.3kJ/mol
��C��s��+O2��g���TCO2��g����H2=-393.5kJ/mol
��H2��g��+$\frac{1}{2}$O2��g���TH2O��l ����H3=-285.8kJ/mol
�ڡ�2+�ۡ�2-�ٿɵã�2C��s��+2H2��g��+O2��g��=CH3COOH��l�������ø�˹���ɼ����ʱ䣬��д�Ȼ�ѧ����ʽ��
��4����֪����C��s��ʯī��+O2��g��=CO2��g����H1=Q1kJ•mol-1
��2CO2��g��+H2 ��g��=C2H2��g��+2O2��g����H2=Q2kJ•mol-1�����ݸ�˹���ɢ١�4+�ڡ�2�õ���4C��s��ʯī��+2H2��g��=2C2H2��g�����ݸ�˹���ɼ����ʱ伴�ɣ�
��� �⣺��1��1mol H2SO4��Һ������ NaOH��Һ��ȫ��Ӧ���ų�114.6kJ��������������2molˮ�ų�114.6kJ����������Ӧ�ķ�Ӧ��Ϊ-114.6kJ/mol��
�к���Ϊ-57.3kJ/mol�����к��ȵ��Ȼ�ѧ����ʽ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
1kg��������ʵ���Ϊ��$\frac{1000g}{58g/mol}$=$\frac{1000}{58}$mol����ȫȼ�����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ5��104kJ����1mol������ȫ��Ӧ����$\frac{58}{1000}$��5��104kJ=2900KJ��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų������������ݷ�Ӧ��Ͳ���״̬��ע�ۼ�״̬�Ͷ�Ӧ���µķ�Ӧ�ȣ���д���Ȼ�ѧ����ʽΪ��C4H10��g��+��$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��H=-2900kJ/mol��
�ʴ�Ϊ��C4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��H=-2900kJ/mol��
��3����֪����CH3COOH��l��+2O2��g���T2CO2��g��+2H2O��l����H1=-870.3kJ/mol
��C��s��+O2��g���TCO2��g����H2=-393.5kJ/mol
��H2��g��+$\frac{1}{2}$O2��g���TH2O��l ����H3=-285.8kJ/mol
���ø�˹���ɽ��ڡ�2+�ۡ�2-�ٿɵã�2C��s��+2H2��g��+O2��g��=CH3COOH��l������H=2����-393.5kJ/mol��+2����-285.8kJ/mol��-��-870.3kJ/mol��=-488.3kJ/mol�������Ȼ�ѧ����ʽΪ2C��s��+2H2��g��+O2��g��=CH3COOH��l������H=-488.3kJ/mol��
�ʴ�Ϊ��2C��s��+2H2��g��+O2��g��=CH3COOH��l������H=-488.3kJ/mol��
��4����֪����C��s��ʯī��+O2��g��=CO2��g����H1=Q1kJ•mol-1
��2CO2��g��+H2 ��g��=C2H2��g��+2O2��g����H2=Q2kJ•mol-1�����ݸ�˹����$\frac{1}{2}$[�١�4+�ڡ�2]�õ���2C��s��ʯī��+H2��g��=C2H2��g������H=��2Q1+Q2�� kJ/mol��
�ʴ�Ϊ��2C��ʯī��s��+H2��g���TC2H2��g����H=��2Q1+Q2�� kJ/mol��
���� ���⿼���Ȼ�ѧ����ʽ����д�����ø�˹���ɵļ��㣬��Ŀ�Ѷ��еȣ�

| A�� | ��̼��ĥ�ɷ�ĩ���Լӿ췴Ӧ���� | |
| B�� | �����¶�һ����Լӿ췴Ӧ���� | |
| C�� | �����������ʱ�������г���N2����Ӧ���ʲ��� | |
| D�� | ����̼�������Լӿ췴Ӧ���� |
| A�� | ��д�Ȼ�ѧ����ʽʱ��ֻҪ�ڻ�ѧ����ʽ���Ҷ�д�������ķ��ź���ֵ���� | |
| B�� | �����ڼ��Ȼ��ȼ�����½��еķ�Ӧ�������ȷ�Ӧ | |
| C�� | ������������ȼ�յ��Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-483.6 kJ | |
| D�� | ������Ӧ���ų������������Ļ�ѧ����ʽ�����Ȼ�ѧ����ʽ |
| A�� | ǿ���ǿ��ķ�Ӧ��һ�����к��� | |
| B�� | 1molC��ȫȼ�շ���383.3kJ�����Ȼ�ѧ����Ϊ��C+O2�TCO2 ��H=-383.3kJ•mol-1 | |
| C�� | ��ϡ��Һ�У��������кͷ�Ӧ����1mol H2Oʱ�ķ�Ӧ�Ƚ����к��� | |
| D�� | ��ʾ�к��ȵ����ӷ���ʽΪ��H+��aq��+OH-��aq���TH2O��l������H=57.3KJ•mol-1 |
| A�� | 1 molҺ̬����������������ȫȼ������ˮ�������ų�642 kJ�������� N2H4��l��+O2��g���TN2��g��+2H2O��g����H=+642 kJ•mol-1 | |
| B�� | 12 gʯīת��ΪCOʱ���ų�110.5 kJ�������� 2C��ʯī��s��+O2��g���T2CO��g����H=-110.5 kJ•mol-1 | |
| C�� | ��֪��H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286 kJ•mol-1�� ��2H2O��l���T2H2��g��+O2��g���ġ�H=+572 kJ•mol-1 | |
| D�� | ��֪N2��g��+3H2��g��?2NH3��g����H=-92.4 kJ•mol-1������һ�����������ܱ������г���0.5 mol N2��g����1.5 mol H2��g����ַ�Ӧ�ų�46.2 kJ������ |
| A�� | ����¯ | B�� | ������ | C�� | ��¯ | D�� | �Ӵ��� |
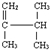 ��֪�Ȼ�ѧ����ʽH+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��
��֪�Ȼ�ѧ����ʽH+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��