��Ŀ����
(18��)MnO2��Zn������ɵ�ص���Ҫԭ�ϣ���ҵ�������̿�(��Ҫ�ɷ�MnO2)����п��(��Ҫ�ɷ�ZnS)��������MnO2��Zn�Ĺ�����������ͼ��ʾ��

��1������I�õ������β��ʵ��������ձ�����������_______ _____(����������)��
��2��ϡ�������ʱ��Ӧ�����ӷ���ʽΪ_______________________________________���÷�Ӧ��������19.2g����A����ת��____________mo1���ӡ�����ʱ��Ӧ���ʽ��������д�ʩ������߽���ʱ��Ӧ���ʵ���________(�����)��
a������ʯ����
b����߽����¶�
c���ʵ���������Ũ��
d���ı����̿�����п��ı���
��3������������Һ�ɵõ�����̼���̣�Ȼ���ڿ���������̼�����Ʊ�MnO2����֪��

д��̼�����ڿ�������������MnO2���Ȼ�ѧ����ʽ_________________________��
��4���ö��Ե缫��������ữ����������Һ�Ʊ�MnO2��װ������ͼ��ʾ��
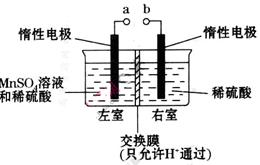
��aӦ��ֱ����Դ��_________(���������)����������
�ڵ������������ӵ�������______________��_____________����ת�Ƶĵ�����Ϊ ����������Һ��
����������Һ�� �ı仯��Ϊ________________��
�ı仯��Ϊ________________��

��1������I�õ������β��ʵ��������ձ�����������_______ _____(����������)��
��2��ϡ�������ʱ��Ӧ�����ӷ���ʽΪ_______________________________________���÷�Ӧ��������19.2g����A����ת��____________mo1���ӡ�����ʱ��Ӧ���ʽ��������д�ʩ������߽���ʱ��Ӧ���ʵ���________(�����)��
a������ʯ����
b����߽����¶�
c���ʵ���������Ũ��
d���ı����̿�����п��ı���
��3������������Һ�ɵõ�����̼���̣�Ȼ���ڿ���������̼�����Ʊ�MnO2����֪��

д��̼�����ڿ�������������MnO2���Ȼ�ѧ����ʽ_________________________��
��4���ö��Ե缫��������ữ����������Һ�Ʊ�MnO2��װ������ͼ��ʾ��

��aӦ��ֱ����Դ��_________(���������)����������
�ڵ������������ӵ�������______________��_____________����ת�Ƶĵ�����Ϊ
 ����������Һ��
����������Һ�� �ı仯��Ϊ________________��
�ı仯��Ϊ________________����1��©��
��2�� ��1.2��d
��1.2��d
��3�� ��H=��a+2b��kJ/mol��
��H=��a+2b��kJ/mol��
��4�������ڲ���������Ӧ��ͨ������Ĥ�����ƶ��γɵ�����1mol
��2��
 ��1.2��d
��1.2��d��3��
 ��H=��a+2b��kJ/mol��
��H=��a+2b��kJ/mol����4�������ڲ���������Ӧ��ͨ������Ĥ�����ƶ��γɵ�����1mol
�����������1������IΪ���ˣ��õ������β��ʵ��������ձ�����������©������2��ϡ�������ʱ��ӦΪMnO2��ZnS��ΪMn2+��Zn2+��ͬʱӦ����S���ʣ��ʷ���ʽΪ��
 ��19.2g����S�����ʵ���Ϊ0.6mol���ɷ�Ӧ����ʽ��֪����1molSת��2mole-,������0.6molSת��1.2mole-���ĸ�ѡ���У�����ʯ���顢��߽����¶ȡ��ʵ���������Ũ�Ⱦ�����߷�Ӧ���ʣ��ʴ�Ϊd����3���ɸ�˹���ɽ���ӦI+II��2�ɵ�
��19.2g����S�����ʵ���Ϊ0.6mol���ɷ�Ӧ����ʽ��֪����1molSת��2mole-,������0.6molSת��1.2mole-���ĸ�ѡ���У�����ʯ���顢��߽����¶ȡ��ʵ���������Ũ�Ⱦ�����߷�Ӧ���ʣ��ʴ�Ϊd����3���ɸ�˹���ɽ���ӦI+II��2�ɵ� ��H=��a+2b��kJ/mol����4���ٸ���װ��ͼ��֪���ҷ���MnSO4��MnO2�ķ�Ӧ��������������Ӧ����aӦ��ֱ����Դ�������������ڵ�����������ΪH+�õ��ӷ�����ԭ��Ӧ���ֽ���Ĥֻ����H+ͨ������H+������Ϊ����������Ӧ��ͨ������Ĥ�����ƶ��γɵ�������ת�Ƶĵ�����Ϊ
��H=��a+2b��kJ/mol����4���ٸ���װ��ͼ��֪���ҷ���MnSO4��MnO2�ķ�Ӧ��������������Ӧ����aӦ��ֱ����Դ�������������ڵ�����������ΪH+�õ��ӷ�����ԭ��Ӧ���ֽ���Ĥֻ����H+ͨ������H+������Ϊ����������Ӧ��ͨ������Ĥ�����ƶ��γɵ�������ת�Ƶĵ�����Ϊ ����1mol�����ݵ���غ����������ʵ����仯Ϊ1mol��
����1mol�����ݵ���غ����������ʵ����仯Ϊ1mol��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2NH3(g)�����ȷ�Ӧ����������������ȷ����
2NH3(g)�����ȷ�Ӧ����������������ȷ���� CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 ��ԭΪN2��һ��ʱ�����Һ�ļ���
��ԭΪN2��һ��ʱ�����Һ�ļ���



 ������������±���ʾ��
������������±���ʾ��

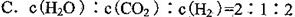


 FeO(s)��CO(g)����H��a kJ��mol��1��ƽ�ⳣ��ΪK��
FeO(s)��CO(g)����H��a kJ��mol��1��ƽ�ⳣ��ΪK��