��Ŀ����
��8�֣�Ϊ�˲ⶨ���ᾧ�壨H2C2O4��xH2O���е�xֵ����������ʵ��
��1����ȡWg���ᾧ�壬���100.00mL��Һ
��2��ȡ25.00mL������Һ����ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L��1��KMnO4��Һ�ζ���KMnO4������ɫΪֹ���������ķ�ӦΪ��
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
�Իش�
��ʵ���У���Ҫ�������У�����ţ� ����ȱ�ٵ������У������ƣ�
E���ձ� F��©�� G����ƿ H�������� I��ҩ�� J����ƿ
��ʵ���У���ҺKMnO4��ҺӦװ�� ʽ�ζ����У���Ϊ
�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ��������յ㣬������õ�x��ֵ�� ����ƫ��ƫС����Ӱ�죩��
���ڵζ�����������ȥamol��L��1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ mol��L��1���ɴˣ�x= ��
�����ζ��յ����ʱ���ӿ̶ȣ�������xֵ�� ����ƫ��ƫС����Ӱ�죩��
��1����ȡWg���ᾧ�壬���100.00mL��Һ
��2��ȡ25.00mL������Һ����ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L��1��KMnO4��Һ�ζ���KMnO4������ɫΪֹ���������ķ�ӦΪ��
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
�Իش�
��ʵ���У���Ҫ�������У�����ţ� ����ȱ�ٵ������У������ƣ�
| A��������ƽ�������롢���ӣ� | B���ζ��� | C��100mL����Ͳ | D��100mL������ƿ |
��ʵ���У���ҺKMnO4��ҺӦװ�� ʽ�ζ����У���Ϊ
�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ��������յ㣬������õ�x��ֵ�� ����ƫ��ƫС����Ӱ�죩��
���ڵζ�����������ȥamol��L��1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ mol��L��1���ɴˣ�x= ��
�����ζ��յ����ʱ���ӿ̶ȣ�������xֵ�� ����ƫ��ƫС����Ӱ�죩��
��ABDEGHI ����ͷ�ιܡ�����̨���ζ��ܼ�
���� ��KMnO4��Һ�и�ʴ�ԣ��ḯʴ��
����Ӱ�� ����ƫ��ƫС����Ӱ�죩��
��
 mol��L��1�� x=
mol��L��1�� x=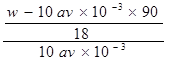 ����
����
�� ƫ�� ����ƫ��ƫС����Ӱ�죩�� (ÿ�գ���)
������������⿼��ʵ����������װ��ʵ�����������������������ʵ����صķ�������㡣ͬʱ���ڵζ���������Ҫע����й�������п��졣�ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڽ��г�ϴ������Ӱ�챾�ζ����ʵ������ʵ���������XֵҲ����ı䡣���ñ����ʺʹ��ⶨ����֮����ڵĵ�����ϵ������������ܣ�������Ŀ�����Ļ�ѧ��Ӧ����ʽ���м��㼴�ɡ�
����������Ľ���˼·���ڲⶨһ�������IJ��ᾧ���в�������ʵ������ٸ��ݲ����Ħ���������ԭ�����ᾧ���в����������ʣ�µľ��ǽᾧˮ�������������������˼·�����ʵ��������ݴ������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
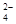 )��c(H��)ԼΪ(����)��
)��c(H��)ԼΪ(����)�� ֮��Ϊ2:1
֮��Ϊ2:1 ��b��
��b�� ��c��
��c�� ��d��
��d�� ������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a
������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a ��Һ�еμ�NaOH��Һ����ҺpH=7����
��Һ�еμ�NaOH��Һ����ҺpH=7����