��Ŀ����
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽ�(���ڳ�����),������ȷ���ǣ� ��
�ٳ����£�pH=1��ǿ����Һ����ˮϡ�ͺ���Һ�и�����Ũ��һ��������
��pH=2�������pH=1�����ᣬ ֮��Ϊ2:1
֮��Ϊ2:1
��pH��ȵ�������Һ��a�� ��b��
��b�� ��c��
��c�� ��d��
��d�� ������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a
������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a
�� ��Һ�еμ�NaOH��Һ����ҺpH=7����
��Һ�еμ�NaOH��Һ����ҺpH=7����
����֪�������ƽ�ⳣ��ΪKa�������ˮ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�������߹�ϵΪ
�ס�������Һ����ǿ����ʣ���֪����Һ��pH������ҺpH����������ס�������Һ�������ϣ����ҺpH���ܵ���7
�ٳ����£�pH=1��ǿ����Һ����ˮϡ�ͺ���Һ�и�����Ũ��һ��������
��pH=2�������pH=1�����ᣬ
 ֮��Ϊ2:1
֮��Ϊ2:1��pH��ȵ�������Һ��a��
 ��b��
��b�� ��c��
��c�� ��d��
��d�� ������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a
������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a��
 ��Һ�еμ�NaOH��Һ����ҺpH=7����
��Һ�еμ�NaOH��Һ����ҺpH=7����
����֪�������ƽ�ⳣ��ΪKa�������ˮ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�������߹�ϵΪ

�ס�������Һ����ǿ����ʣ���֪����Һ��pH������ҺpH����������ס�������Һ�������ϣ����ҺpH���ܵ���7
| A���ۢݢ� | B���ۢܢ� | C���ܢݢ� | D���٢ڢ� |
A
�������������ȷ��ǿ����Һȫ�����룬��ˮϡ�ͺ���Һ�и�����Ũ��һ�������ͣ��ڴ���pH=2�������pH=1�����ᣬ
 ֮��Ϊ1:10������ȷ�������ε�ˮ��Һ���Լ��ԣ�ͬŨ�ȣ�����ǿ��˳��Ϊd>b>c>a,��pH��ȵ�������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a���ܴ���
֮��Ϊ1:10������ȷ�������ε�ˮ��Һ���Լ��ԣ�ͬŨ�ȣ�����ǿ��˳��Ϊd>b>c>a,��pH��ȵ�������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a���ܴ��� ��Һ�еμ�NaOH��Һ����ҺpH=7����c(Na) < 2c(SO42��)������ȷ������ȷ����3a=14ʱ�����ҺpH=7�����ϣ���������ѡ��ΪA��
��Һ�еμ�NaOH��Һ����ҺpH=7����c(Na) < 2c(SO42��)������ȷ������ȷ����3a=14ʱ�����ҺpH=7�����ϣ���������ѡ��ΪA�����������⿼��������Һ��֪ʶ����ȷ�������ʵĸ������Һ��֪ʶ������ͱȽϼ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

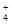 ����c��SO
����c��SO ��֮���� ( )
��֮���� ( )