��Ŀ����
17��ʵ��װ�ü�ʵ������ǻ�ѧʵ��Ļ������ݣ���1��ijͬѧ����Ͳ��ȡ��Һ����������Ͳ��Һ��İ�Һ����ʹ�����ˮƽ������Ϊ15.0������������
��Һ����Ӱ�Һ�����͵㣬����Ϊ9.0���������ѧ��ʵ�ʵ�����Һ�������Χ�Ǵ���6ml��
��2��������ƽ�ľ�ȷ����0.1g����������ƽ��ȡ10.5g������Ʒ��1g����ʹ�����룩ʱ��������Ʒ
��������ƽ�����̣���������Ʒ��ʵ������Ϊ9.5g��
��3���ڡ������ᴿ����ʵ���У�����õ������������ܽ⡢���ˡ��������������У����������������
�ֱ��ǽ��裻����������ʹ���Ⱦ��ȣ�
��4��Ҫ����������������
A������ʳ��ˮ��ɳ��
B����KNO3��NaCl�Ļ��Һ�л��KNO3
C��ˮ�����͵Ļ����
D��CCl4Һ����ױ�Һ��Ļ�����֪CCl4��ױ����ܣ��е�ֱ�Ϊ76.75���110.6�棩
�ٷ���A��B��D��������IJ����ǹ��ˡ���ȴ�ᾧ������
�ڷ���C������ʱ��ʹ����������һ�������������ʱ����ʹ�ã����������Ƿ�Һ©����
�������������ʱ��Ҫʹ�þƾ��Ƶ���B��D������ţ���
���� ��1��ʹ����Ͳ����Һ������������ʱ����Ҫ��Һ����Һ�����ʹ���ƽ����������ʾ����С����������ʾ������
��2����������ƽ��������ʱ����ȷ�IJ������������룬����ŷ��ˣ���m��=m��-m�����ݴ˼��㣻
��3�������ᴿ��ͨ���ܽ⣨�Ѳ�������ʳ�γ������룩�����ˣ��Ѳ����ﳹ�׳�ȥ����������ʳ�δ���Һ�з���������õ�ʳ�εĹ��̣���ת�ƣ������˳��Ϊ�ܽ�-����-����-ת�ƣ��ݴ˷��������������ã����н�ɣ�
��4��A������ʳ��ˮ��ɳ�ӣ�ɳ�Ӳ�����ˮ��
B��NaCl���ܽ�����¶�Ӱ�첻��KNO3���ܽ�����¶�Ӱ���
C��ˮ�����͵Ļ����ֲ㣻
D��CCl4Һ����ױ�Һ��Ļ������ܣ����е㲻ͬ���Դ������
��� �⣺��1����������Һ����Ӱ�Һ�����ʹ�����ʣ��Һ����������ʣ��Һ����������С��9�����ģ����ѧ��ʵ�ʵ�������Һ������Ǵ���15������ȥ9�����IJ�ֵ��Ҳ���Ǵ���6�������ʴ�Ϊ������6mL��
��2��������ƽ��ȷ��0.1g����������ƽ��������ʱ����ȷ�IJ������������룬����ŷ��ˣ���m��=m��-m����������Ϊ10.5gʱ��ʹ�õ����������Ϊ10g�����������Ϊ0.5g����ҩƷ������m=10.0g-0.5g=9.5g���ʴ�Ϊ��0.1��9.5��
��3���ڡ������ᴿ����ʵ���У���������Ϊ�ܽ�-����-����-�ᾧ�����ܽ⡢���ˡ�������ת�Ʋ����ж�Ҫ�õ��IJ��������Dz����������ܽ�����в������������ǽ��裬�ӿ��ܽ⣻�ڹ��˲����в�����������������������ʱ�������������ǽ��裬��ֹ�ֲ��¶ȹ��ߣ����Һ�ηɽ������ʴ�Ϊ�����裻����������ʹ���Ⱦ��ȣ�
��4��A������ʳ��ˮ��ɳ�ӣ�ɳ�Ӳ�����ˮ�������ù��˷��룬����Ҫ���ȣ�
B��NaCl���ܽ�����¶�Ӱ�첻��KNO3���ܽ�����¶�Ӱ�����������ȴ�ᾧ�����룬�ܽ�ʱ��Ҫ���ȵõ��ȱ�����Һ��
C��ˮ�����͵Ļ����ֲ㣬���÷�Һ�����룬��Ҫ��Һ©����
D��CCl4Һ����ױ�Һ��Ļ������ܣ����е㲻ͬ�����������룬��Ҫ�ƾ��Ƽ��ȣ�
�ٷ���ABD�IJ����ֱ�Ϊ���ˡ���ȴ�ᾧ�����ʴ�Ϊ�����ˣ���ȴ�ᾧ������
��������������֪������Cʱʹ�õ���Ҫ�����Ƿ�Һ©�����ʴ�Ϊ����Һ©����
�۷���ʱ��ʹ�þƾ��Ƶ���BD���ʴ�Ϊ��BD��
���� ���⿼����������ᴿ��Ϊ��Ƶ���㣬�������ʵ����ʡ����ʲ��켰�������뷽��Ϊ���Ĺؼ������ػ������뷽��ѡ��Ŀ��飬��Ŀ�ѶȲ���

| A�� | �٢� | B�� | �٢ۢ� | C�� | �ۢڢ� | D�� | �ۢڢ٢� |
| A�� | ԭ������֮��Ϊ2������Ԫ�ز�����λ��ͬһ���� | |
| B�� | D-������36�����ӣ���Ԫ��Dλ�ڵ������ڵڢ�A�� | |
| C�� | λ��ͬһ����ļ�������Ԫ�أ���ԭ������Ϊx�����ҵ�ԭ����������Ϊx+4 | |
| D�� | λ��ͬһ���ڵļ�������Ԫ�أ���λ�ڵڢ�A�壬ԭ������Ϊx����λ�ڵڢ�A�壬����ԭ����������Ϊx+19 |
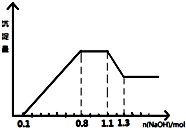 ij��Һ��ֻ���ܺ���H+��NH4+��Mg2+��Al3+��Fe3+��CO32-��SO42-��NO3-�еļ��֣���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������
ij��Һ��ֻ���ܺ���H+��NH4+��Mg2+��Al3+��Fe3+��CO32-��SO42-��NO3-�еļ��֣���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | ��Һ�е�������ֻ��H+��Mg2+��Al3+���ܺ���Fe3+ | |
| B�� | ��Һ��һ������CO32-��NO3-��һ������SO42- | |
| C�� | ��Һ��c��NH4+��=0.3 mol/L | |
| D�� | c��H+����c��Al3+����c��Mg2+��=1��1��1 |
| A�� | 0.4 mol | B�� | 0.5 mol | C�� | 0.6 mol | D�� | 0.8 mol |
| A�� | �������ϩ | B�� | ����ͱ�ϩ | C�� | �������ϩ | D�� | ����ͱ�ϩ |