��Ŀ����
18��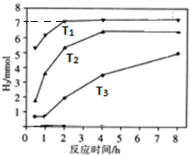 ��Ȼ������Ҫ�ɷּ��飩��������������[���⡢�ʻ���COS��������C2H5SH��]������������������Һϴ�ӳ�ȥ��
��Ȼ������Ҫ�ɷּ��飩��������������[���⡢�ʻ���COS��������C2H5SH��]������������������Һϴ�ӳ�ȥ����1����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
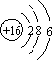 ���ʻ�����ӵĵ���ʽΪ
���ʻ�����ӵĵ���ʽΪ ��
����2������˵����ȷ����bd��
a��������Է������������Ҵ�������е����
b��ͬ�¶�ͬŨ����Na2CO3��Һ��pH����Na2SO4��Һ��˵����Ԫ�طǽ�����ǿ��̼Ԫ��
c��H2S���Ӻ�CO2���Ǽ��Է��ӣ���Ϊ���Ƕ���ֱ���η���
d�������һ���Ӱ�죬������������H2S
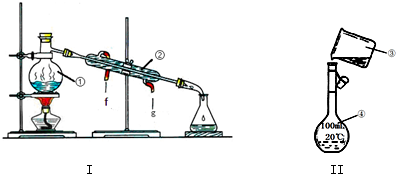
��3���ʻ���������������Һ���������õĹ������£����ֲ�������ȥ����
COS$��_{I}^{NaOH��Һ}$Na2S��Һ$��_{��}^{��}$X��Һ+H2
�ٷ�ӦI���������������⣬����ˮ���ɣ��仯ѧ����ʽΪCOS+4NaOH=Na2S+Na2CO3+2H2O��
����֪X��Һ����Ԫ�ص���Ҫ������ʽΪS2O32-���������Ҫ��Ӧ�����ӷ���ʽΪ2S2-+5H2O=S2O32-+4H2��+2OH-��
����ͼ�Ƿ�Ӧ���У��ڲ�ͬ��Ӧ�¶��£���Ӧʱ����H2�����Ĺ�ϵͼ��Na2S��ʼ����Ϊ3mmo1����
a���ж�T1��T2��T3�Ĵ�С��T1��T2��T3��
b����T1�¶��£���ַ�Ӧ����X��Һ�г�S2O32-�⣬������������Ӧ��ͬʱ������SO42-������Һ��c��S2O32-����c��SO42-��=5��2��
���� ��1��S��������Ϊ16��ԭ�Ӻ�����3�����Ӳ㣬��������Ϊ6���ʻ�������������̼���ӽṹ���ƣ���Ϊֱ���ͣ�
��2��a���Ҵ��к������
b��ͬ�¶�ͬŨ����Na2CO3��Һ��pH����Na2SO4��Һ����֪��Ӧ��ۺ���������ԣ�
c��CO2�ṹ�Գƣ�������������غϣ�
d�������һ���Ӱ�죬�����ѵ���������ӣ�
��3���ٷ�ӦI���������������⣬����ˮ���ɣ���Ԫ���غ��֪����������ΪNa2S��Na2CO3��
��������ˮ��Ӧ����S2O32-���������������ƣ����ݵ����غ��ԭ���غ���д��
��a����ͼ��֪���¶ȸߵķ�Ӧ���ʴ���Ӧ��ʱ��̣�
b.3molNa2S��ֻ����S2O32-ת��12mol���ӣ�T1�¶��£����ɵ�����Ϊ7mol��ת�Ƶ���Ϊ14mol����ϵ����غ���㣮
��� �⣺��1��S��������Ϊ16��ԭ�Ӻ�����3�����Ӳ㣬��������Ϊ6����ԭ�ӽṹʾ��ͼΪ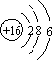 ���ʻ�������������̼���ӽṹ���ƣ���Ϊֱ���ͣ������ʽΪ
���ʻ�������������̼���ӽṹ���ƣ���Ϊֱ���ͣ������ʽΪ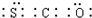 ��
��
�ʴ�Ϊ��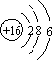 ��
��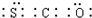 ��
��
��2��a���Ҵ��к���������Ҵ��е����������a����
b��ͬ�¶�ͬŨ����Na2CO3��Һ��pH����Na2SO4��Һ����֪��Ӧ��ۺ����������Ϊ���̼�ᣬ����Ԫ�طǽ�����ǿ��̼Ԫ�أ���b��ȷ��
c��CO2�ṹ�Գƣ�������������غϣ���Ϊ�Ǽ��Է��ӣ�Ϊֱ�߽ṹ����H2S����V�ͼ��Է��ӣ���c����
d�������һ���Ӱ�죬�����ѵ���������ӣ�������������H2S����d��ȷ��
�ʴ�Ϊ��bd��
��3���ٷ�ӦI���������������⣬����ˮ���ɣ���Ԫ���غ��֪����������ΪNa2S��Na2CO3����ӦΪCOS+4NaOH=Na2S+Na2CO3+2H2O��
�ʴ�Ϊ��COS+4NaOH=Na2S+Na2CO3+2H2O��
��������ˮ��Ӧ����S2O32-���������������ƣ��䷴Ӧ�����ӷ���ʽΪ��2S2-+5H2O=S2O32-+4H2��+2OH-���ʴ�Ϊ��2S2-+5H2O=S2O32-+4H2��+2OH-��
��a����ͼ��֪���¶ȸߵķ�Ӧ���ʴ���Ӧ��ʱ��̣���T1��T2��T3���ʴ�Ϊ��T1��T2��T3��
b.3molNa2S��ֻ����S2O32-ת��12mol���ӣ�T1�¶��£����ɵ�����Ϊ7mol��ת�Ƶ���Ϊ14mol���������SO42-Ϊx���ɵ����غ��֪x��8+��3-x����4=14�����x=0.5mol����n��S2O32-��=$\frac{3-0.5}{2}$=1.25mol����Һ��c��S2O32-����c��SO42-��=1.25��0.5=5��2��
�ʴ�Ϊ��5��2��
���� ���⿼����ۺϣ��漰���ʽṹ�����ʡ�������ԭ��Ӧ���㡢ͼ����������ӷ�Ӧ�ȣ����ط�Ӧԭ���и�Ƶ����Ŀ��飬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

 | 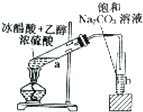 |
| A����֤����������������ѧ��Ӧ | B��ʵ������ȡ�������� |
 |  |
| C����ȡ�����ʵĴֱ����� | D���ᴿ�����ʵĹ�ҵ�ƾ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 �T2CuI��+13I2+��K2SO4+��
�T2CuI��+13I2+��K2SO4+�� �����ɵ�I2�պ�����n mol S2O32-��S2O32-������ΪS4O62-������μӷ�Ӧ��Cu��IO3��2���ʵ���Ϊ��������
�����ɵ�I2�պ�����n mol S2O32-��S2O32-������ΪS4O62-������μӷ�Ӧ��Cu��IO3��2���ʵ���Ϊ��������| A�� | $\frac{n}{10}$ mol | B�� | $\frac{n}{11}$ mol | C�� | $\frac{n}{12}$ mol | D�� | $\frac{n}{13}$ mol |
| ѡ�� | ���� | ���� |
| A | ��ǿ�������ȡ������ | ��������Һ����ȡ���� |
| B | ��Ӧ��Ũ��Խ��Ӧ����Խ�� | �����£�����ͬ����Ƭ�ֱ����������Ũ��ϡ���ᣬŨ��������Ƭ���ܽ��� |
| C | �ṹ��������Ƶ����ʣ��е�����Է���������������� | NH3�е����PH3 |
| D | �ܽ��С�ij��������ܽ�ȸ�С�ij���ת�� | AgCl�����еμ�Na2S��Һ���Եõ�Ag2S���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 0.27 s | B�� | 0.44 s | C�� | 1.33 s | D�� | 2 s |
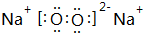 ��д��M2Z2��ˮ��Ӧ�����ӷ���ʽ��2Na2O2+2H2O=4Na++4OH-+O2����
��д��M2Z2��ˮ��Ӧ�����ӷ���ʽ��2Na2O2+2H2O=4Na++4OH-+O2����
 Ϊ2Cu2++3OH-+Cl-=Cu2��OH��3Cl����������4.29g Cu2��OH��3Cl���������Ϻ������Ϊ0.448L����״������
Ϊ2Cu2++3OH-+Cl-=Cu2��OH��3Cl����������4.29g Cu2��OH��3Cl���������Ϻ������Ϊ0.448L����״������