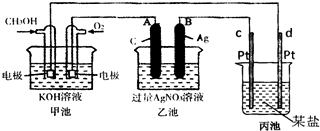
��Ŀ����
3���Ͻ��ѳ�Ϊ�ɻ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС���ͬѧ��Ϊ�ⶨij��þ3%��5%����þ�Ͻ𣨲�������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհף���ʵ�鷽��һ������þ�Ͻ�������NaOH��Һ��Ӧ���ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
��ʵ�鲽�衿
��1����ȡ5.4g��þ�Ͻ��ĩ��Ʒ������VmL 2.0mol/LNaOH��Һ�У���ַ�Ӧ����NaOH��Һ�����V��97mL��
��2�����ˡ�ϴ�ӡ�����������壮�ò�������δϴ�ӹ��壬���þ������������ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��ʵ�鷽������
����þ�Ͻ�������ϡ������Һ��Ӧ���ⶨ����������ͨ��״����Լ20�棬1.01105Pa���������
���������ۡ�
��3��ͬѧ����ѡ��ͼ1ʵ��װ�����ʵ�飺
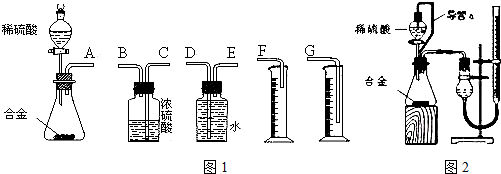
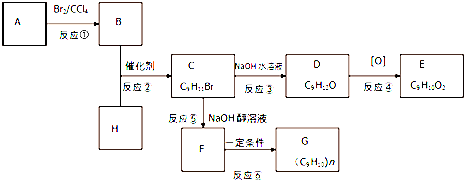
������Ϊ�����װ��������˳���ǣ�A��E��D��G����ӿ���ĸ���ɲ���������
��ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ���������������������һ�����ϡ����Ҳ����˳��������ƿ�������������ԭ��þ������ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ���
��ʵ�����ʱ���ڶ�ȡ����ʵ�����������������ʱ������Ϊ��������ACD��
A����ʵ��װ����ȴ���ٶ���
B�������ƶ���ͲF��ʹ����Һ������ƿ��Һ����ƽ
C�������ƶ���ͲG��ʹ����Һ������ƿ��Һ����ƽ
D�������밼Һ�����͵�ˮƽ����ȡ��Ͳ��ˮ�����
��4����ϸ����ʵ��װ�ú�ͬѧ�Ǿ�������Ϊ�������������ϴ���ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС���������������ͼ2��ʾ��ʵ��װ�ã�
��װ���е���a�������DZ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
��ʵ��ǰ���ʽ�ζ�����Һ������ֱ�ΪV1mL��V2mL����������������ΪV1-V2mL��
���� ����һ����������������Һ��Ӧ����ƫ��������������
��1��þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
����������1����װ�õ���װ˳�Ͻ���ˮ��Ӧ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ��������������������������Ͳ�ڵ���Ӧ������Ͳ�ײ���
��þ������ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ���
�۷�Ӧ���ȵ����������¶�ƫ�ߣ���Ӧ��ȴ���ٽ��ж�ȡ�������������ȡʵ�����������������ʱ�����ƶ���Ͳ��ʹ����Һ������ƿ��Һ����ƽ�������밼Һ�����͵�ˮƽ��ȡ�����������
��2���ٱ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ڵζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ�������������ע��Ӧ���ָ������ζ�����Һ��ȸߣ����ռ�������ζ�����Һ��������������С��
��� �⣺����һ����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��1����þΪ3%ʱ���������ĺ�����ߣ�5.4g�Ͻ�����������Ϊ��5.4g����1-3%��=5.4��97%g����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 2mol
5.4g��97%g V��10-3L��2.0mol/L
����54g����5.4g��97%g��=2mol����V��10-3L��2.0mol/L������ã�V=97����V��NaOH��Һ����97mL��
�ʴ�Ϊ��97mL��
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��þ����������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
����������1����װ�õ���װ˳�Ͻ���ˮ��Ӧ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽���������������ѹǿԭ����ˮ�ų�����Ͳ��ˮ��������������������������Ͳ�ڵ���Ӧ������Ͳ�ײ���������˳��Ϊ����A���ӣ�E����D���ӣ�G����
�ʴ�Ϊ��E��D��G��
��þ������ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ����������˳��������ƿ��
�ʴ�Ϊ��þ������ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ���
�۷�Ӧ���ȵ����������¶�ƫ�ߣ���Ӧ��ȴ���ٽ��ж�ȡ�������������ȡʵ�����������������ʱ�����ƶ���Ͳ��ʹ����Һ������ƿ��Һ����ƽ�������밼Һ�����͵�ˮƽ��ȡ�����������
��ѡACD��
��2����װ���е���a�������ǣ����ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ʴ�Ϊ�����ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ڵζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ��������������ռ�������ζ�����Һ�������С���ʲⶨ���������ΪV1-V2��
�ʴ�Ϊ��V1-V2��
���� ���⿼�����ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�| A�� | ����ͨ�����ȵ�CuO��ĩ | B�� | ������̼ͨ��Na2O2��ĩ | ||
| C�� | ���������������·�����Ӧ | D�� | ��п��Ͷ��FeCl2��Һ |

 ��Ȼ������Ҫ�ɷּ��飩��������������[���⡢�ʻ���COS��������C2H5SH��]������������������Һϴ�ӳ�ȥ��
��Ȼ������Ҫ�ɷּ��飩��������������[���⡢�ʻ���COS��������C2H5SH��]������������������Һϴ�ӳ�ȥ��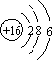 ���ʻ�����ӵĵ���ʽΪ
���ʻ�����ӵĵ���ʽΪ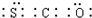 ��
��
 ��
�� ��
�� ��
��