��Ŀ����
����Ŀ��ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��
������0.50 mol/L NaOH��Һ
��ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����_____g��
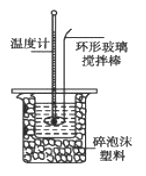
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����_____________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | |||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | ||
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g�����������к��ȡ�H=______________ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�______��
A��ʵ��װ�ñ��¡�����Ч����
B����ȡNaOH��Һ�����ʱ���Ӷ���
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
���𰸡�5.01/2H2SO4(aq����NaOH(aq����1/2Na2SO4(aq����H2O(l�� ��H����57.3kJ/mol4.0-53.5kJ/molacd
��������
���⿼��һ�����ʵ���Ũ����Һ���Ƶ��йؼ��㣬�Ȼ�ѧ����ʽ����д�Լ��к��ȵļ��㣬�������ȣ���Ŀ�ѶȲ���ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�������Լ��ⶨ��Ӧ�ȵ��������⡣
I����1������ʵ������û��245mL����ƿ��Ӧѡ��250mL������ƿ������250mL����Һ��������������ƹ��������m=cVM=0.50mol/L��0.25L��40g/mol=5.0g��
II����1�������к��ȵĶ��壬ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������ϡ�������������ϡ��Һ�ֱ���ǿ�ᡢǿ���÷�Ӧ���Ȼ�ѧ����ʽΪ1/2H2SO4(aq����NaOH(aq����1/2Na2SO4(aq����H2O(l�� ��H����57.3kJ/mol��
��2�������ݱ����ṩ����Ϣ����2���������ϴ���ȥ�����¶Ȳ�ƽ��ֵ=��4.0+3.9+4.1����3=4.0��C����50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80mL��1g/mL=80g���¶ȱ仯��ֵΪ��T=4����������0.025molˮ�ų�������ΪQ=mc��T=80g��4.18J/��g������4.0��=1337.6J����1.3376kJ������ʵ���õ��к�����H=-1.3376kJ��0.025mol=-53.5 kJ/mol��
��a��ʵ��װ�ñ��¡�����Ч������ã�����ᵼ������ɢʧ��ʹ�ⶨ���ƫС��a����ȷ��b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ������57.3kJ/mol��b�����c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У����ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ᵼ������ɢʧ��c��ȷ��d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�Ҫ���¶ȼƻ����ٲⶨH2SO4��Һ���¶ȣ�����������²��С��d����ȷ����ѡacd��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ʵ����ģ��ϳ�������������£�

����ͼ��ѡ����ȡ����ĺ���װ�ã�

��1��װ��C������Ϊ_________________��ʵ����ͨ������װ��C�Ʊ�_________��
A��H2 B��H2S C��CO2 D��O2
��2��ʵ������װ��D�Ʊ�NH3�Ļ�ѧ����ʽΪ_______________________________��
��3������װ��B�Ʊ�SO2������ѡ���Լ�Ϊ___________��
A��Ũ���ᡢ�������ƹ��� B��Ũ���ᡢͭƬ
C��ϡ���ᡢ��������Һ D��Ũ���ᡢ��м
��4��SO2��O2ͨ����װ�ã���װ�õ����ó��˿��Կ���SO2��O2�������⣬������__________��

��5������һ������SO2(g)��O2(g)�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У��ڲ�ͬ�¶��½��з�Ӧ�õ����±��е��������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | ||
SO2 | O2 | SO2 | O2 | ||
1 | T1 | 4 | 2 | x | 0.8 |
2 | T2 | 4 | 2 | 0.4 | y |
����x=_______mol��y=_______mol��T1______T2���>������=����<��)
��6��SO2β������NaOH��Һ���գ��õ�Na2SO3��NaHSO3�����Ρ���һ�����ʵ�����SO2������������Һ��Ӧ��������Һ��Na2SO3��NaHSO3�����ʵ���֮��Ϊ2:3����μӷ�Ӧ��SO2��NaOH�����ʵ���֮��Ϊ________��
A. 5:7 B��1:2 C��9:4 D��9:13
��7�������백ˮ��Ӧ��������炙�������泥��ֳ�ȡ(NH4)2SO4��NH4HSO4�������Ʒ7.58 g�����뺬0.1 molNaOH����Һ��������Ӧ����OH-+H+��H2O, ��OH-+NH4+��NH3��+H2O����ַ�Ӧ������1792 mL����״��������֪�����ȷ�Ӧ������Ʒ��(NH4)2SO4�����ʵ���Ϊ_______mol��NH4HSO4�����ʵ���Ϊ_______mol��

