��Ŀ����
����Ŀ�����������ʾ��ij���е���Ҫ������Ⱦ��ΪSO2��NOx��CO�ȣ�����Ҫ��ԴΪȼú��������β�������������о���
��1��Ϊ����ȼú��SO2���ŷţ��ɽ�úת��Ϊ���ȼ��ˮú����CO��H2����
��֪��![]() ��H��241.8kJ��mol��1��
��H��241.8kJ��mol��1��
![]() ��H����110.5kJmol��1
��H����110.5kJmol��1
д����̿��1molˮ������Ӧ����ˮú�����Ȼ�ѧ����ʽ��________��
��2������β����NO���ڷ��������������ɵģ���ӦΪN2��g����O2��g��![]() 2NO��g�� ��H��0��
2NO��g�� ��H��0��
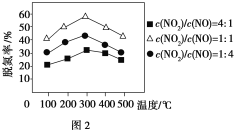
������0.8molN2��0.2molO2�����ƿ�����ɣ��Ļ���������ij�ܱ������У�����1300����Ӧ�ﵽƽ�⣬�������8��10��4molNO��������¶��´˷�Ӧ�Ļ�ѧƽ�ⳣ��K��________������Ƽ���������
�������������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
��3��������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�������������ų�����Һ����NO2��

���缫A�ĵ缫��ӦʽΪ________��
�缫B�ĵ缫��ӦʽΪ________��
�����������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ����SO32�����÷�Ӧ�����ӷ���ʽΪ________��
���𰸡�C��s����H2O��g����CO��g����H2��g�� ��H����131.3kJ��mol��14��10��6�¶����ߣ���Ӧ���ʼӿ���ƽ�������ƶ�SO2��2H2O��2e����SO42����4H��2HSO3����2H����2e����S2O42����2H2O4S2O42����2NO2��8OH����8SO32����N2��4H2O
��������(1)����֪����2H2(g)+O2(g)=2H2O(g)��H=-483.6KJ/mol����C(s)+![]() O2(g)�TCO(g)����H=-110.51kJmol-1�����ø�˹���ɣ�����-����
O2(g)�TCO(g)����H=-110.51kJmol-1�����ø�˹���ɣ�����-����![]() �ɵã�C(s)+H2O(g)=CO(g)+H2(g)����H=(-110.5kJ/mol)-(-241.8kJ/mol)=+13l.3 kJ/mol�����Խ�̿��ˮ������Ӧ���Ȼ�ѧ����ʽΪ��C(s)+H2O(g)�TCO(g)+H2(g)����H=+13l.30kJmol-1��
�ɵã�C(s)+H2O(g)=CO(g)+H2(g)����H=(-110.5kJ/mol)-(-241.8kJ/mol)=+13l.3 kJ/mol�����Խ�̿��ˮ������Ӧ���Ȼ�ѧ����ʽΪ��C(s)+H2O(g)�TCO(g)+H2(g)����H=+13l.30kJmol-1��
(2)���跴Ӧ�����������aL����������ã�
N2(g)+O2(g)![]() 2NO(g)
2NO(g)
��ʼŨ�� ![]()
![]() 0
0
ת��Ũ�� ![]()
![]()
![]()
ƽ��Ũ�� ![]() -
- ![]()
![]() -
- ![]()
![]()
���������ʵ�Ũ�ȴ������ʽ��K=![]() ��4��10-6��
��4��10-6��
�������з����Ļ�ѧ��Ӧ��N2(g)+O2(g)![]() 2NO(g)��H��0�����Ǹ����ȷ�Ӧ���¶����ߣ���Ӧ���ʼӿ죬ƽ�����������ƶ�����λʱ���ڲ�����NO�ࣻ
2NO(g)��H��0�����Ǹ����ȷ�Ӧ���¶����ߣ���Ӧ���ʼӿ죬ƽ�����������ƶ�����λʱ���ڲ�����NO�ࣻ
(3)������ͼʾ��֪��������������Ϊ����������Զ����������ڵ�A��Ϊ��������������������ӦSO2-2e-+2H2O�TSO42-+4H+��B��Ϊ��������HSO3������ԭΪS2O42���������ĵ缫��ӦΪ2HSO3����2H����2e����S2O42����2H2O��
�������ų�����ҺΪS2O42-�������������䷢����Ӧ��S2O

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�����Ŀ��ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��
������0.50 mol/L NaOH��Һ
��ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����_____g��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����_____________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | |||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | ||
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g�����������к��ȡ�H=______________ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�______��
A��ʵ��װ�ñ��¡�����Ч����
B����ȡNaOH��Һ�����ʱ���Ӷ���
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
����Ŀ����ͬԪ�ص�ԭ���ڷ������������ӵ�������С����һ����ֵ x ����ʾ���� x Խ����ԭ��������������Խǿ�������γɵķ����г�Ϊ������ɵ�һ����
������ijЩ������Ԫ�ص� x ֵ��
Ԫ�ط��� | Li | Be | B | C | N | F |
x ֵ | 0.98 | 1.57 | 2.04 | 2.25 | 2.89 | 3.98 |
Ԫ�ط��� | Na | Mg | Si | P | S | Cl |
x ֵ | 0.93 | 1.35 | 1.90 | 2.19 | 2.58 | 3.16 |
��1��ͨ������ x ֵ�仯���ɣ�ȷ��Al��N�� x ֵ��Χ��________�� x (Al)��________
��2���Ʋ� x ֵ��ԭ�Ӱ뾶��ϵ��________�����ݶ�����Ԫ�ص� x ֵ�仯�ص㣬������Ԫ�����ʵ�________�仯���ɡ�
��3��ij�л�������ṹʽΪ�� ������SO��������Ϊ���õ��Ӷ�ƫ��˭��________��дԭ�����ƣ���
������SO��������Ϊ���õ��Ӷ�ƫ��˭��________��дԭ�����ƣ���
��4��������ɸ������ǣ����ɼ�����ԭ����ӦԪ�ص� x ��ֵ(�� x )�� x ��1.7ʱ��һ��Ϊ���Ӽ������� x ��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlF 3 �л�ѧ��������________��
��5��Ԥ��Ԫ�����ڱ��У� x ֵ��С��Ԫ�ص�λ�ã�_______��������Ԫ�س��⣩��