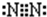��Ŀ����
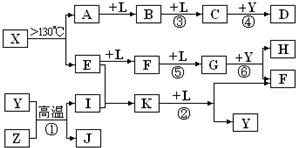
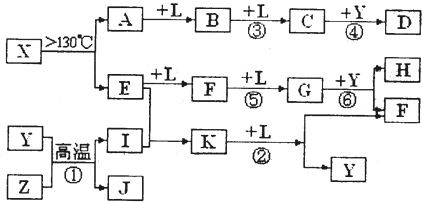
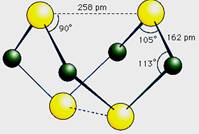
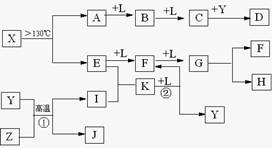
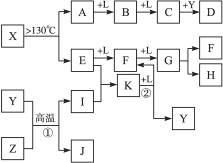
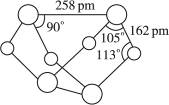
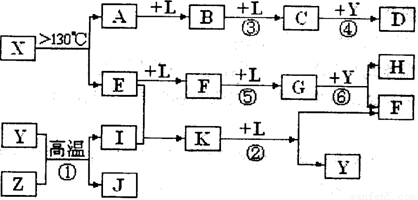
��10�֣���֪A��E��I��L�dz����ķǽ������ʣ�����AΪ����ɫ���壻Z�dz����Ľ������ʣ�B����Է���������A��32��C����Է���������B��16��Y��һ�ֳ�����Һ�壬J�Ǵ�����������D��H��K������Ҫ�Ļ�����Ʒ��X�ǽṹ�д�̽����һ�����ͷ��ӣ������Ԫ�ص����ԭ���������18,���п�ͼ�в��ַ�Ӧ��������ȥ��
�Իش��������⣺
��1��E�ĽṹʽΪ___________���ڷ�Ӧ�١��ڡ��ۡ��ܡ��ݡ��������ڷ�������ԭ��Ӧ����___________��������ţ�
��2��д����Ӧ�ڵĻ�ѧ����ʽ:_________________________________________
��3������G��F�Ļ������ͨ�������ռ���Һ������ȫ�������գ�������G��F�����ʵ���֮��Ӧ����n��G���Un��F��____________________
��4��J�����H��ϡ��Һ��Ӧ�����ӷ���ʽΪ___________________________________��
��5��t��ʱ����2mol E��1mol Iͨ�����Ϊ2L�ĺ����ܱ������з�����Ӧ��2min��ﵽƽ��n��K����0��2mol, ��ʱ�����¶Ȳ��䣬������룬��E�����������α仯____________������������䡱������С����
��1��N��N , �ܣ���1�֣���2�֣�
��2��4NH3 + 5O2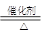 4NO +6H2O��2�֣�
4NO +6H2O��2�֣�
��3����1��1��2�֣�
��4��3Fe3O4 + 28H+ + NO3- = 9Fe3+ + NO��+ 14H2O��2�֣� ������2�֣�
�����������⿼�����ƶϡ�AΪ����ɫ���壬��Ϊ���ʣ���֪Ϊ��B����Է���������A��32�������Ƴ�BΪSO2��C����Է���������B��16��CΪSO3��J�Ǵ��������Ҫ�ɷ֣�J��Fe3O4���ӿ�ͼ��֪��L��O2��Y��һ�ֳ�����Һ�壬C��Y������Ҫ������ƷD����֪Y��H2O��D��H2SO4���ɷ�Ӧ�ٵ������Ͳ���J���Բ²ⷴӦ��Ϊ3Fe+4H2O(g) Fe3O4+4H2��Z��Fe��I��H2��H��K������Ҫ�Ĺ�ҵ��Ʒ����E��F��G֮���ת����������֪E��N2��N2��H2��Ӧ����K��K��NH3����Ӧ����NH3��O2�Ĵ�����������NO��H2O�����Ͽ�ͼ��F��NO��NO��O2������Ӧ����NO2��G��NO2��NO2��ˮ��Ӧ���������NO��H�����ᡣ�����A��E���Լ�X�ǽṹ�д�̽����һ�����ͷ��ӣ������Ԫ�ص����ԭ���������18, �ɵ�X��S4N4��
Fe3O4+4H2��Z��Fe��I��H2��H��K������Ҫ�Ĺ�ҵ��Ʒ����E��F��G֮���ת����������֪E��N2��N2��H2��Ӧ����K��K��NH3����Ӧ����NH3��O2�Ĵ�����������NO��H2O�����Ͽ�ͼ��F��NO��NO��O2������Ӧ����NO2��G��NO2��NO2��ˮ��Ӧ���������NO��H�����ᡣ�����A��E���Լ�X�ǽṹ�д�̽����һ�����ͷ��ӣ������Ԫ�ص����ԭ���������18, �ɵ�X��S4N4��
��1��N2���������Ե��ӣ��ṹʽΪN��N���ڷ�Ӧ�١��ڡ��ۡ��ܡ��ݡ��������ڷ�������ԭ��Ӧ���Ǣ�SO3+H2O=H2SO4û�е��ӵ�ʧ����2��NH3�������з�������������ѧ����ʽΪ4NH3 + 5O2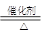 4NO +6H2O����3��NO2��NO�������ͨ�������ռ���Һ������ȫ�������գ�ת��Ϊ�����κ��������Σ�ֻҪNO2�����ʵ�������NO�����ʵ�������ȫ�����գ���n(NO2)��n(NO)��1��1�������ķ�ӦΪ2NO2+2NaOH=NaNO2+NaNO3+H2O��NO2+NO+2NaOH=2NaNO2+H2O����4��Fe3O4�������ϡ���ᷴӦ������������NO��ˮ�����ӷ���ʽΪ3Fe3O4+28H++NO3-=9Fe3++
NO��+14H2O����5���ϳɰ�����Ӧ��һ�������С�ķ�Ӧ��������룬�൱��ѹǿ����һ����ѹǿ����ƽ��������Ӧ�����ƶ���NH3�������������
4NO +6H2O����3��NO2��NO�������ͨ�������ռ���Һ������ȫ�������գ�ת��Ϊ�����κ��������Σ�ֻҪNO2�����ʵ�������NO�����ʵ�������ȫ�����գ���n(NO2)��n(NO)��1��1�������ķ�ӦΪ2NO2+2NaOH=NaNO2+NaNO3+H2O��NO2+NO+2NaOH=2NaNO2+H2O����4��Fe3O4�������ϡ���ᷴӦ������������NO��ˮ�����ӷ���ʽΪ3Fe3O4+28H++NO3-=9Fe3++
NO��+14H2O����5���ϳɰ�����Ӧ��һ�������С�ķ�Ӧ��������룬�൱��ѹǿ����һ����ѹǿ����ƽ��������Ӧ�����ƶ���NH3�������������

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�