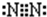��Ŀ����
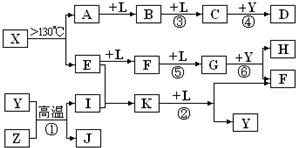
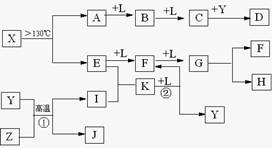
��֪A��E��I��L�dz����ķǽ������ʣ�����AΪ����ɫ���壻Z�dz����Ľ������ʣ�B����Է���������A��32��C����Է���������B��16��Y��һ�ֳ�����Һ�壬J�Ǵ�����������D��H��K������Ҫ�Ļ�����Ʒ��X�ǽṹ�д�̽����һ�����ͷ��ӣ������Ԫ�ص����ԭ���������18�����п�ͼ�в��ַ�Ӧ��������ȥ��
�Իش��������⣺
��1��E�ĽṹʽΪ ���ڷ�Ӧ�١��ڡ��ۡ��ܡ��ݡ��������ڷ�������ԭ��Ӧ���� ��������ţ�
��2��д����Ӧ�ڵĻ�ѧ����ʽ��
��3������G��F�Ļ������ͨ�������ռ���Һ������ȫ�������գ�������G��F�����ʵ���֮��Ӧ����n��G����n��F��
��4��J�����H��ϡ��Һ��Ӧ�����ӷ���ʽΪ ��
��5��t��ʱ����2mol E��1mol Iͨ�����Ϊ2L�ĺ����ܱ������з�����Ӧ��2min��ﵽƽ��n��K��=0.2mol����ʱ�����¶Ȳ��䣬������룬��E�����������α仯 ������������䡱������С����

�Իش��������⣺
��1��E�ĽṹʽΪ
��2��д����Ӧ�ڵĻ�ѧ����ʽ��
��3������G��F�Ļ������ͨ�������ռ���Һ������ȫ�������գ�������G��F�����ʵ���֮��Ӧ����n��G����n��F��
��4��J�����H��ϡ��Һ��Ӧ�����ӷ���ʽΪ
��5��t��ʱ����2mol E��1mol Iͨ�����Ϊ2L�ĺ����ܱ������з�����Ӧ��2min��ﵽƽ��n��K��=0.2mol����ʱ�����¶Ȳ��䣬������룬��E�����������α仯
������J�Ǵ���������ΪFe3O4��Y��һ�ֳ�����Һ�壬Z�dz����Ľ������ʣ��ɷ�Ӧ�ٿ�֪��ZΪFe��YΪH2O��IΪH2��A��E��I��L�dz����ķǽ������ʣ�����AΪ����ɫ���壬AΪS���ʣ���A
B
C����B����Է���������A��32��C����Է���������B��16������֪BΪSO2��CΪSO3��LΪO2�����C+Y��D��֪��DΪH2SO4��
��X��A+E��AΪS���ʣ���X����SԪ�أ����X������Ԫ�ص����ԭ���������18��E�dz����ķǽ������ʣ���EԪ�ص����ԭ������Ϊ32-18=14����EΪN2����X���ӵ����ģ�Ϳ�֪��XΪS4N4����E
F
G��֪��FΪNO��GΪNO2����G
H+F��֪HΪHNO3����E+I��K�����EΪN2��IΪH2����֪KΪNH3��K
F+YΪNH3+O2��NO+H2O������ת����ϵ���ݴ˽��
| L |
| L |
��X��A+E��AΪS���ʣ���X����SԪ�أ����X������Ԫ�ص����ԭ���������18��E�dz����ķǽ������ʣ���EԪ�ص����ԭ������Ϊ32-18=14����EΪN2����X���ӵ����ģ�Ϳ�֪��XΪS4N4����E
| ���� |
| ���� |
| ˮ |
| ���� |
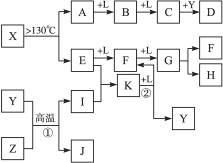
����⣺J�Ǵ���������ΪFe3O4��Y��һ�ֳ�����Һ�壬Z�dz����Ľ������ʣ��ɷ�Ӧ�ٿ�֪��ZΪFe��YΪH2O��IΪH2��A��E��I��L�dz����ķǽ������ʣ�����AΪ����ɫ���壬AΪS���ʣ���A
B
C����B����Է���������A��32��C����Է���������B��16������֪BΪSO2��CΪSO3��LΪO2�����C+Y��D��֪��DΪH2SO4��
��X��A+E��AΪS���ʣ���X����SԪ�أ����X������Ԫ�ص����ԭ���������18��E�dz����ķǽ������ʣ���EԪ�ص����ԭ������Ϊ32-18=14����EΪN2����X���ӵ����ģ�Ϳ�֪��XΪS4N4����E
F
G��֪��FΪNO��GΪNO2����G
H+F��֪HΪHNO3����E+I��K�����EΪN2��IΪH2����֪KΪNH3��K
F+YΪNH3+O2��NO+H2O������ת����ϵ��
��1��EΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ���ṹʽΪ��N��N��
��Ӧ����Fe+H2O��Fe3O4+H2������������ԭ��Ӧ����Ӧ����NH3+O2��NO+H2O������������ԭ��Ӧ��
��Ӧ����SO2+O2��SO3������������ԭ��Ӧ����Ӧ����SO3+H2O��H2SO4�����ڷ�������ԭ��Ӧ��
��Ӧ����NO+O2��NO2������������ԭ��Ӧ����Ӧ����NO2+H2O��HNO3+NO������������ԭ��Ӧ��
�����ڷ�������ԭ��Ӧ���� �ܣ�
�ʴ�Ϊ��N��N���ܣ�
��2����Ӧ���ǰ�����������Ӧ����һ��������ˮ����Ӧ����ʽΪ��4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6 H2O��
��3������NO��NO2�Ļ������ͨ�������ռ���Һ������ȫ�������գ�������ӦNO+NO2+2NaOH=2NaNO2+H2O��������n��NO2����n��NO����1��1��
�ʴ�Ϊ����1��1��
��4���������ǿ�����ԣ�Fe3O4����ϡ������Һ��Ӧ��������������NO��ˮ��Fe3O4��+2��+3���������ʵ���֮��Ϊ1��2����Ӧ���ӷ���ʽΪ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��
�ʴ�Ϊ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��
��5��t��ʱ����2mol N2��1molH2ͨ�����Ϊ2L�ĺ����ܱ������з�����Ӧ��N2��g��+3H2��g��?2NH3��g����2min��ﵽƽ�⣬n��NH3��=0.2mol����ʱ�����¶Ȳ��䣬������룬ѹǿ��������ӦΪ���������С�ķ�Ӧ����ƽ��������Ӧ�����ƶ������ڷ�Ӧ1mol��������������ܵ����ʵ������˼���2mol���ʵ�����ƽ��ʱ�����������������
�ʴ�Ϊ������
| L |
| L |
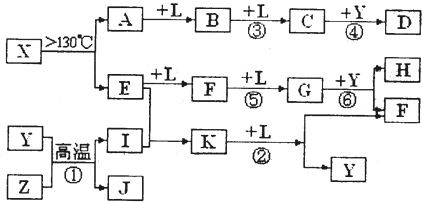
��X��A+E��AΪS���ʣ���X����SԪ�أ����X������Ԫ�ص����ԭ���������18��E�dz����ķǽ������ʣ���EԪ�ص����ԭ������Ϊ32-18=14����EΪN2����X���ӵ����ģ�Ϳ�֪��XΪS4N4����E
| ���� |
| ���� |
| ˮ |
| ���� |
��1��EΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ���ṹʽΪ��N��N��
��Ӧ����Fe+H2O��Fe3O4+H2������������ԭ��Ӧ����Ӧ����NH3+O2��NO+H2O������������ԭ��Ӧ��
��Ӧ����SO2+O2��SO3������������ԭ��Ӧ����Ӧ����SO3+H2O��H2SO4�����ڷ�������ԭ��Ӧ��
��Ӧ����NO+O2��NO2������������ԭ��Ӧ����Ӧ����NO2+H2O��HNO3+NO������������ԭ��Ӧ��
�����ڷ�������ԭ��Ӧ���� �ܣ�
�ʴ�Ϊ��N��N���ܣ�
��2����Ӧ���ǰ�����������Ӧ����һ��������ˮ����Ӧ����ʽΪ��4NH3+5O2
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |
��3������NO��NO2�Ļ������ͨ�������ռ���Һ������ȫ�������գ�������ӦNO+NO2+2NaOH=2NaNO2+H2O��������n��NO2����n��NO����1��1��
�ʴ�Ϊ����1��1��
��4���������ǿ�����ԣ�Fe3O4����ϡ������Һ��Ӧ��������������NO��ˮ��Fe3O4��+2��+3���������ʵ���֮��Ϊ1��2����Ӧ���ӷ���ʽΪ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��
�ʴ�Ϊ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��
��5��t��ʱ����2mol N2��1molH2ͨ�����Ϊ2L�ĺ����ܱ������з�����Ӧ��N2��g��+3H2��g��?2NH3��g����2min��ﵽƽ�⣬n��NH3��=0.2mol����ʱ�����¶Ȳ��䣬������룬ѹǿ��������ӦΪ���������С�ķ�Ӧ����ƽ��������Ӧ�����ƶ������ڷ�Ӧ1mol��������������ܵ����ʵ������˼���2mol���ʵ�����ƽ��ʱ�����������������
�ʴ�Ϊ������
����������������ͼ�����ʽ����N��S��Fe��Ԫ�ص��ʼ��仯����֮����ת����ϵ����������������ѧ�������д��������ѧƽ�����ȣ���AΪ����ɫ���嵥�ʣ�Y��һ�ֳ�����Һ�壬J�Ǵ������������Ⱦ�Ϊ����ͻ�ƿڣ����ת����ϵ����˳�����������������жϣ���4�������ӷ���ʽ����д���״��㣬�ѵ㣬�����Է���������ԭ��Ӧ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ