��Ŀ����
����Ŀ��(1)���Ѿ���SO2���ʹ����Ϊ0.25g��L��1��ȡ300.00mL���Ѿƣ�ͨ���ʵ��ķ���ʹ����SO2ȫ���ݳ�����H2O2����ȫ������ΪH2SO4��Ȼ����0.0900mol��L��1NaOH����Һ���еζ���
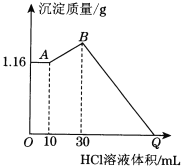
�ٵζ�ǰ������ʱ��Ӧѡ����ͼ�е�___(�����)

������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�����������Һ������___(�����)��
��=10mL ��=40mL ��<10 mL ��>40 mL��
�������ζ�ʵ�������ζ��յ�ʱ��Һ��pH=8.8����ѡ���ָʾ��Ϊ______��ѡ���ָʾ��ʱ����жϷ�Ӧ����ζ��յ㣺______��
�ܵζ����յ�ʱ������NaOH��Һ25.00mL�������Ѿ���SO2����Ϊ___g��L��1���ζ��յ����ʱ���ӿ̶��ߣ�����������ʵ��ֵ______������ƫ��������ƫ����������Ӱ��������
(2)ijѧ����0.100molL-1��KOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ��
A.��ȡ20mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��
B.�ñ���Һ��ϴ�ζ���2��3�Σ�
C.��ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ���ʹ���촦������Һ��
D.ȡ��KOH��Һע���ʽ�ζ������̶���0������2��3cm ����
E.����Һ������0������0�����¿̶ȣ����¶�����
F.����ƿ���ڵζ������棬�ñ�KOH��Һ�ζ����յ㲢���¿̶ȡ�
ʵ���� | KOH��Һ��Ũ��(mol/L) | �ζ����ʱ��KOH��Һ��������(mL) | ����������Һ�����(mL) |
1 | 0.10 | 22.62 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
����ȷ���������˳���ǣ��������ĸ��д��_________��
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ_______��������λ��Ч���֣���
���𰸡��� �� ��̪ ���������һ�α���Һ����Һǡ�ñ�dz��ɫ�����ڰ�������ޱ仯 0.24 ƫ�� B��D��C��E��A��F 0.11mol/L
��������
(1)�ټ�����Һ�ü�ʽ�ζ���ʢ�ţ�NaOH�Ǽ���Һ��ʢ���ڼ�ʽ�ζ��ܣ�
�ڸ��������Ľṹ�ж���Һ�������С��
�۸���ָʾ���ı�ɫ��Χ����ϸ���ָʾ���ڲ�ͬ��pH��ʾ����ɫ�жϵζ��յ㣻
�ܸ��ݷ�Ӧ��SO2+H2O2=H2SO4��H2SO4+2NaOH=Na2SO4+2H2O�ɵù�ϵʽ�����ݹ�ϵʽ2NaOH��H2SO4��SO2�����������Ƶ����ʵ������������������������ټ���������Ѿ��еĶ��������������ݶ��������ı�����Һ�����Ӱ�죬�����SO2�����ʵ�����ϵ������
(2)���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ��Ȳ�����
���ȼ������ĵı�NaOH��Һ�������Ȼ�����ǡ�÷�Ӧʱn(NaOH)=n(HCl)���������Ũ�ȡ�
(1)�ټ�����Һ�ü�ʽ�ζ���ʢ�ţ���NaOH����Һ�ü�ʽ�ζ���ʢ�ţ��ų����ݵķ����Ǣۣ�
������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�������ζ��ܵ�0�̶����Ϸ���10mL�̶����·�����40mL�п̶ȵ���Һ������ζ���50mL�̶�������Һ�壬��˹��ڵ�Һ�����>(50.00mL-10.00mL)=40.00mL������ѡ��ܺ�����
�������ζ�ʵ��������֪Ũ�ȵļ����ζ�δ֪Ũ�ȵ�����Һ�����ζ��յ�ʱ��Һ��pH=8.8�����ڷ�̪�ı�ɫ��Χ��8.2��10.0�����ȵı�ɫ��Χ��3.1��4.4������Ӧ��ѡ���ָʾ��Ϊ��̪��ָʾ��������Һ�У���ʼ����ɫ�����ﵽ�ζ��յ�ʱ��Һ��Ϊdz��ɫ������ѡ���ָʾ��ʱ������Ӧ����ζ��յ㣬���������һ�α���Һ����Һǡ�ñ�dz��ɫ�����ڰ�������ޱ仯��
�ܸ��ݷ�����Ӧ�Ļ�ѧ����ʽSO2+H2O2=H2SO4��H2SO4+2NaOH=Na2SO4+2H2O�ɵù�ϵʽ2NaOH��H2SO4��SO2����֪SO2������m(SO2)=![]() n(NaOH)��M(SO2)=
n(NaOH)��M(SO2)=![]() ��0.0900mol/L��0.025L��64g/mol=0.072g�����Ը����Ѿ��еĶ���������Ϊ��
��0.0900mol/L��0.025L��64g/mol=0.072g�����Ը����Ѿ��еĶ���������Ϊ��![]() =0.24g/L��
=0.24g/L��
���ڵζ��յ����ʱ���ӿ̶��ߣ������ı�NaOH��Һ�����ƫС��n(NaOH)ƫС�����ʹ�ò��������ʵ��ֵƫ�ͣ�
(2)�ٲ����IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ���������ȷ˳��Ϊ��B��D��C��E��A��F��
���������ݾ���Ч��ƽ������V(KOH��Һ)=![]() mL=22.71mL������c(��)=
mL=22.71mL������c(��)= =0.11mol/L��
=0.11mol/L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�