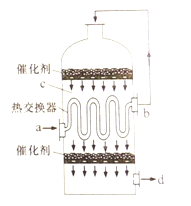
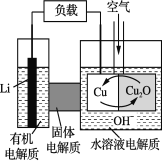
��Ŀ����
����Ŀ�����м�������ȷ���ǣ� ��
A.��25gCuSO4��5H2O����ͭ�����ܽ���975gˮ�У�������Һ���ʵ���������Ϊ2.5%
B.0.23gNa��9.77gH2O��ȫ��Ӧ��������Һ���ʵ���������Ϊ2.3%
C.7.8gNa2O2��92.2gH2O��ȫ��Ӧ��������Һ���ʵ���������Ϊ8.0%
D.32.2gNa2SO4��10H2O����ˮ���500 mL��Һ�У����ӵ����ʵ���Ũ��Ϊ0.6mol��L��1
���𰸡�D
��������
A. ��25gCuSO4��5H2O����ͭ�����ܽ���975gˮ�У�������Һ����ΪCuSO4������Ϊ16g���������ʵ���������Ϊ1.6%��A����
B. 0.23gNa��9.77gH2O��ȫ��Ӧ��������Һ������ΪNaOH������Ϊ0.4g��ͬʱ����ˮ����Һ������С��10.0g���������ʵ�������������4.0%��B����
C. 7.8gNa2O2��92.2gH2O��ȫ��Ӧ��������Һ�����ʵ�����Ϊ8.0g����Һ������С��100g���������ʵ�������������8.0%��C����
D. 32.2gNa2SO4��10H2O����ˮ���500 mL��Һ�У�Na+��SO42-�����ʵ���Ũ�Ⱥ�Ϊ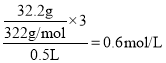 ��D��ȷ��
��D��ȷ��
��ѡD��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ