题目内容
已知:O2(g) = O2+(g)+e- 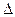 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e-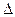 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S)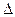 H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6 (s)的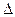 H是
H是
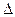 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1PtF6-(g) =PtF6(g)+e-
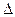 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1O2+(g)+PtF6-(g) =O2PtF6(S)
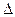 H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1则反应O2(g)+PtF6(g) = O2PtF6 (s)的
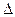 H是
H是| A.77.6 kJ | B.-77.6 kJ·mol-1 | C.+77.6kJ·mol-1 | D.-886.8kJ·mol-1 |
B
略

练习册系列答案
 新课标阶梯阅读训练系列答案
新课标阶梯阅读训练系列答案
相关题目
 。
。
 一是丁烷。在101 kPa时,10 kg丁烷(相对分子质量为58)完全燃烧生成CO2和液态H2O放出热量5×105 kJ,丁烷的燃烧热为___________,丁烷燃烧的热化学方程式为_________________________________________________。
一是丁烷。在101 kPa时,10 kg丁烷(相对分子质量为58)完全燃烧生成CO2和液态H2O放出热量5×105 kJ,丁烷的燃烧热为___________,丁烷燃烧的热化学方程式为_________________________________________________。 FeO(s) ?
FeO(s) ? 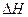 =-272.0KJ/mol
=-272.0KJ/mol 则 Al(s)的单质和FeO(s)反应的热化学方程式是_____________________________________。
则 Al(s)的单质和FeO(s)反应的热化学方程式是_____________________________________。 CH3OH (g) △H 1(反应Ⅰ)
CH3OH (g) △H 1(反应Ⅰ)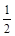 O2(g)=CO2(g) △H 2=-283 kJ·mol-
O2(g)=CO2(g) △H 2=-283 kJ·mol- 1 (反应Ⅱ)
1 (反应Ⅱ)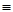 O
O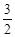 O2(g)=CO2(g)+2H2O(g) △H4
O2(g)=CO2(g)+2H2O(g) △H4
 学式)
学式)