��Ŀ����
16��X��Y��Z����Ԫ�أ�ԭ���������μ�С��X�ǵ�����������Ԫ�أ��䲿�ֵ�������ͼ1��ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Zԭ�Ӽ۵����Ų�ʽnsnnpn���ش��������⣺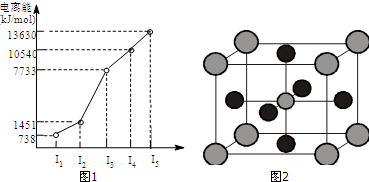
��1��Xԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p64s2����XԪ�ع��ɵĵ������ڽ������壮
��2���縺�ԣ�X��Y�����������=����������
��3��Ԫ��Z��һ���⻯���ѧʽΪZ2H4������Ҫ�Ļ���ԭ�ϣ��й�Z2H4���ӵ�˵����ȷ����BCD������ĸ����
A������������
B����ʹ���Ը��������Һ��ɫ��
C������5���Ҽ���1���м�
D��Z��ԭ������ԭ���γɵĻ�ѧ��������ת
��4��X�����������ѣ�Ti��������������ã����γ������Σ��侧��ṹʾ��ͼ��ͼ2��ʾ��X��Ti��O����Ԫ�ض�Ӧ�����ӷֱ�λ������������ġ���������ģ���
�þ���Ļ�ѧʽΪ��CaTiO3��
�Ʋ�þ������������Щ�������ʣ�BC
A����̬ʱ�ܵ��磮 B���ۻ�״̬�ܵ��磮C���нϸߵ��۵㣮 D��Ӳ�Ⱥ�С��
���� X��Y��Z����Ԫ�أ�ԭ���������μ�С��X�ǵ������ڵ�����Ԫ�أ���ͼ���䲿�ֵ����ܿ�֪������������ܾ�������X����+2�ۣ����ڢ�A�壬��XΪCa��Zԭ�Ӽ۵����Ų�ʽΪnsnnpn��s�ܼ�ֻ������2�����ӣ���n=2����۵����Ų�ʽΪ2s22p2����ZΪCԪ�أ�X��YԪ�ؾ�����ͬ����������ϼۣ�����ͬ���壬Y��ԭ����������̼Ԫ�أ���YΪMg���ݴ˽��
��� �⣻X��Y��Z����Ԫ�أ�ԭ���������μ�С��X�ǵ������ڵ�����Ԫ�أ���ͼ���䲿�ֵ����ܿ�֪������������ܾ�������X����+2�ۣ����ڢ�A�壬��XΪCa��Zԭ�Ӽ۵����Ų�ʽΪnsnnpn��s�ܼ�ֻ������2�����ӣ���n=2����۵����Ų�ʽΪ2s22p2����ZΪCԪ�أ�X��YԪ�ؾ�����ͬ����������ϼۣ�����ͬ���壬Y��ԭ����������̼Ԫ�أ���YΪMg��
��1��XΪCa��ԭ�Ӻ��������Ϊ20����ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p64s2�����ڽ������壬�ʴ�Ϊ��1s22s22p63s23p64s2��������
��2��ͬ�������϶��µ�һ�����ܼ�С���ʵ�һ�����ܣ�Ca��Mg���ʴ�Ϊ������
��3��CԪ�ص�һ���⻯��C2H4�������в��������������Cԭ��֮���γ�C=C˫����Hԭ����Cԭ��֮���γ�C-H����ʹ���Ը��������Һ��ɫ������5���Ҽ���1���м���C-H��������������ת����A����BCD��ȷ���ʴ�Ϊ��BCD��
��4��Ca�����������ѣ�Ti��������������ã����γ������Σ�Ca��Ti��O����Ԫ�ض�Ӧ�����ӷֱ�λ�ھ�������������ġ���������ģ��Զ����Ti�����о�����֮�����������λ�������ϣ�ÿ��Ti����Ϊ12���湲�ã��������Ӻ���Χ12������������ڣ�
������Ca������Ŀ=1��Ti������Ŀ=8��$\frac{1}{8}$=1��O������Ŀ=6��$\frac{1}{2}$=3���ʻ�ѧʽΪCaTiO3���þ����������Ӿ��壬��̬ʱ�����ܵ��磬�ۻ�״̬�ܵ��磬�нϸߵ��۵㣬Ӳ�Ƚϴ�
�ʴ�Ϊ��CaTiO3��BC��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ��縺�ԡ���ѧ������������ȣ��Ѷ��еȣ�ע������������뻯�ϼ۹�ϵ��

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�| A�� | �ǻ��ĵ���ʽ 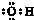 | B�� | ȩ���Ľṹ��ʽ-COH | ||
| C�� | 1-��ϩ�ļ���ʽ  | D�� | �۱�ϩ�Ľṹ��ʽ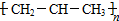 |
| A�� | ���飾���飾���� | B�� | 1-�����飼1-�ȱ��� | ||
| C�� | һ�����飾һ������ | D�� | �����飾�����飾������ |
| A�� | ���ǵ�ԭ�Ӻ�����Ӳ�����˵���������Ӷ����� | |
| B�� | ԭ�ӵ���������������7�� | |
| C�� | ���ǵĵ��ʵ���ɫ��˵���������Ӷ����� | |
| D�� | ���ǵ��⻯����ȶ�����˵���������Ӷ���ǿ |
| A�� | ԭ�Ӱ뾶�������Ա仯 | |
| B�� | Ԫ�صĻ��ϼ۳������Ա仯 | |
| C�� | Ԫ��ԭ�ӵĺ�������Ų��������Ա仯 | |
| D�� | ��һ�����ܳ������Ա仯 |
| A�� | ����Ԫ����ǽ���Ԫ�����γɹ��ۻ����� | |
| B�� | Cl-��S2-��Ca2+��K+�뾶��С | |
| C�� | Ŀǰʹ�õ�Ԫ�����ڱ��У�������ں���36��Ԫ�� | |
| D�� | �����ԭ���У�����˽Ͻ����������˶��ĵ��������ϸ� |
 ��ͼ��ʾ��Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼���£���Ƭ�ϵ������������ǣ�������
��ͼ��ʾ��Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼���£���Ƭ�ϵ������������ǣ�������