��Ŀ����
7��ijú����Ҫ����Al2O3��SiO2�����Ʊ���ʽ������[Al2��SO4��3•2Al��OH��3]��Һ����������������SO2���ŷţ����Ʊ����̣�
��֪��25�棬Ksp��CaCO3��=2.8��10-9��Ksp��CaSO4��=9.1��10-6��
��1�������ٵ����ƹ��ˣ�
��2�����ʱ��Ӧ�����ӷ���ʽΪAl2O3+6H+=2Al3++3H2O��Ϊ��������ʱ��Ԫ�صĽ����ʣ��ɲ�ȡ�Ĵ�ʩ��ú�����飬���裨ʹ��ú����������Һ��ֽӴ�������������Ũ�ȣ�д2������
��3������2�Ļ�ѧʽ��CaSO4���Է�������2���ɵ���Ҫԭ�������ӷ���ʽ������ּ�Ҫ˵����CaCO3+2H+=Ca2++H2O+CO2����������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4��������Ca2++SO42-=CaSO4�� ��CaCO3+2H+=Ca2++H2O+CO2�����ٽ�CaCO3��s�������ܽ�ƽ�����ܽⷽ���ƶ���������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4�����ٽ�CaSO4��s�������ܽ�ƽ������������ƶ�����
��4����ʽ��������Һ����SO2����Al2��SO4��3•Al2��SO3��3���������Һͨ����������������һ�������Σ������Ʊ���ʽ��������Һ��ѭ��ʹ�ã���д���йط�Ӧ�Ļ�ѧ����ʽ��Al2��SO4��3•2Al��OH��3+3SO2=Al2��SO4��3•Al2��SO3��3+3H2O��2Al2��SO4��3•Al2��SO3��3+3O2=4Al2��SO4��3��
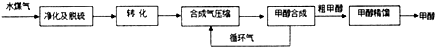
���� ú����Ҫ����Al2O3��SiO2����ú���м���ϡ���ᣬ������ӦAl2O3+6H+=2Al3++3H2O��SiO2����Ӧ��Ȼ����ù��˷����õ�����1ΪSiO2����Һ������Ϊϡ�����Al2��SO4��3��������ҺpHΪ3.6��Ȼ�����CaCO3��ĩ��������ӦCaCO3+2H+�TCa2++CO2��+H2O��CaSO4Ϊ�����������2�ijɷ���ҪΪCaSO4�����˵���Һ��
��1�����������Թ������Һ���ù��˷�����
��2����������������������ܺ�ϡ���ᷴӦ�����κ�ˮ��Ϊ��������ʱ��Ԫ�صĽ����ʣ����Բ���������ҺŨ�ȡ������¶Ȼ�����Ӧ��Ӵ�����ȷ�����
��3������2�Ļ�ѧʽ��CaSO4��������Ũ��������c��Ca2+��•c��SO42-����Ksp��CaSO4����
��4��Al2��SO4��3•2Al��OH��3��SO2��Ӧ����Al2��SO4��3•Al2��SO3��3��Al2��SO4��3•Al2��SO3��3��O2��������Al2��SO4��3��
��� �⣺ú����Ҫ����Al2O3��SiO2����ú���м���ϡ���ᣬ������ӦAl2O3+6H+=2Al3++3H2O��SiO2����Ӧ��Ȼ����ù��˷����õ�����1ΪSiO2����Һ������Ϊϡ�����Al2��SO4��3��������ҺpHΪ3.6��Ȼ�����CaCO3��ĩ��������ӦCaCO3+2H+�TCa2++CO2��+H2O��CaSO4Ϊ�����������2�ijɷ���ҪΪCaSO4�����˵���Һ��
��1�����������Թ������Һ���ù��˷�����������������ϡ���ᡢ��������������ϡ���ᣬ���Բ��ù��˷������룬�ʴ�Ϊ�����ˣ�
��2����������������������ܺ�ϡ���ᷴӦ�����κ�ˮ�����ӷ�Ӧ����ʽΪAl2O3+6H+=2Al3++3H2O��Ϊ��������ʱ��Ԫ�صĽ����ʣ����Բ���������ҺŨ�ȡ������¶Ȼ�����Ӧ��Ӵ�����ȷ�����
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O����ú�����飬���裨ʹ��ú����������Һ��ֽӴ������ʵ��ӳ������ʱ�䣬��������Ũ�ȣ������¶ȣ�
��3������2�Ļ�ѧʽ��CaSO4��CaCO3+2H+=Ca2++H2O+CO2����������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4��������Ca2++SO42-=CaSO4�� ��CaCO3+2H+=Ca2++H2O+CO2�����ٽ�CaCO3��s�������ܽ�ƽ�����ܽⷽ���ƶ���������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4�����ٽ�CaSO4��s�������ܽ�ƽ������������ƶ���
�ʴ�Ϊ��CaSO4��CaCO3+2H+=Ca2++H2O+CO2����������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4��������Ca2++SO42-=CaSO4�� ��CaCO3+2H+=Ca2++H2O+CO2�����ٽ�CaCO3��s�������ܽ�ƽ�����ܽⷽ���ƶ���������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4�����ٽ�CaSO4��s�������ܽ�ƽ������������ƶ���
��4��Al2��SO4��3•2Al��OH��3��SO2��Ӧ����Al2��SO4��3•Al2��SO3��3��Al2��SO4��3•Al2��SO3��3��O2��������Al2��SO4��3����Ӧ����ʽΪAl2��SO4��3•2Al��OH��3+3SO2=Al2��SO4��3•Al2��SO3��3+3H2O��2Al2��SO4��3•Al2��SO3��3+3O2=4Al2��SO4��3��
�ʴ�Ϊ��Al2��SO4��3•2Al��OH��3+3SO2=Al2��SO4��3•Al2��SO3��3+3H2O��2Al2��SO4��3•Al2��SO3��3+3O2=4Al2��SO4��3��
���� ���⿼�����ʷ�����ᴿ��Ϊ��Ƶ���㣬���ؿ������ӷ�Ӧ���������ܽ�ƽ�⼰������������ȷ���ʵ����ʼ����������������ɽ���ѵ��ǣ�3���⣬֪��Ũ�Ȼ����ܶȻ��Ĺ�ϵ����Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | c��NH4+��=2c��SO42-����c��H+��=c��OH-�� | B�� | c��NH4+����c��SO42-����c��H+����c��OH-�� | ||
| C�� | c��SO42-����c��NH4+����c��H+����c��OH-�� | D�� | c��SO42-����c��NH4+����c��OH-����c��H+�� |
ij�о���ѧϰС��Ϊ�ⶨ��ˮ��Ũ�ȣ����ð�ˮ���ᴿ����ʱ���Լ��������������������ʵ�����£�
�������ϣ�
�ټ��ȵı�ɫ��Χ��pH��3.1��ɫ��pH=3.1��4.4��ɫ��pH��4.4��ɫ
�ڷ�̪�ı�ɫ��Χ��pH��8.2��ɫ��pH=8.2��10.0�ۺ�ɫ��pH��10.0��ɫ
����֪��Fe3+��Fe2+��Cu2+ת��Ϊ��������ʱ��Ӧ��pH���±�1��
| Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | 5.2 |
| ��ȫ����ʱ��pH | 3.7 | 9.6 | 6.4 |
| ��1 | |||
| ��� | 1 | 2 | 3 | 4 | |
| �������/mL | 25.05 | 25.00 | 26.80 | 24.95 | |
ʵ��һ���궨��ˮ��Ũ��
ȡ25.00mLԼΪ0.10mol•L-1��ˮ����ƿ�У���0.1000mol•L-1������еζ���ʵ�������������ϱ�2��ʾ��
��1���ζ�����ˮ������ӷ���ʽΪNH4Cl+H2O?NH3��H2O+HCl���ɴ˿���֪ѡ��ĵζ�ָʾ��ӦΪ���ȣ�������ȡ���̪����
��2���ð�ˮ��ȷŨ��Ϊ0.1000mol•L-1������ȷ��С�������λ��
��3�����3����Һ������Ũ���ɴ�С��˳��Ϊc��Cl-����c��NH4+����c��H+����c��OH-����
ʵ��� �ᴿ��������
ijѧϰС��ͬѧ��Ӻ�FeSO4��Fe2��SO4��3���ʵ�CuSO4��Һ���ᴿ����������Ҫʵ�鲽�����£�
��һ�� �����Һ�м���3% H2O2��Һ��ַ�Ӧ���ټ���ϡ��ˮ������ҺpH�����ˣ�
�ڶ��� ����Һ�м���ϡ���������ҺpH ��1��2���ᴿ������
��4������3% H2O2��Һ�������ǽ�Fe 2+����ΪFe 3+��
��5����ϡ��ˮ����pHӦ������Χ3.7-5.2֮�䣮
��6���������ʿ��������ϡ��ˮ����BC��������ĸ��
A��NaOH B��Cu��OH��2 C��CuO D��NaHCO3��
��ע����ȥˮ�������ˮú����55��59%��H2��15��18%��CO��11��13%��CO2��������H2S��CH4����ȥH2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ���������ɽ��м״��ϳɣ�
��1����CH4ת����CO����ҵ�ϳ����ô�ת���������䷴Ӧԭ��Ϊ��
CH4 ��g��+$\frac{3}{2}$O2 ��g��?CO��g��+2H2O ��g����H=-519KJ•mol-1����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
��X��T1��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Y��T2��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Z��T3��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
��֪��T1��T2��T3������������Ϣ������Ϊ��������Ӧ��ѡ������˴�����Z���X����Y����Z������ѡ��������Ǵ����Ըߡ��ٶȿ졢��Ӧ�¶Ƚϵͣ�
��2���ϳ�����ѹ�����º����10m3�״��ϳ������ڴ��������£����м״��ϳɣ���Ҫ��Ӧ�ǣ�2H2��g��+CO��g��?CH3OH��g����H=-181.6kJ•mol-1��T4���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | H2 | CO | CH3OH |
| Ũ��/��mol•L-1�� | 0.2 | 0.1 | 0.4 |
��������ͬ�����CO��H2����T5�淴Ӧ��10 min��ﵽƽ�⣬��ʱc��H2��=0.4 mol•L-1�����ʱ���ڷ�Ӧ����v��CH3OH��=0.03mol•��L•min��-1��
��3�����������У��ϳ���Ҫ����ѭ������Ŀ�������ԭ��CO��H2�������ʣ�����߲�����������ɣ�����
��4����ͼ1Ϊ��þ-�������Ρ�ȼ�ϵ��ԭ��ʾ��ͼ���缫Ϊþ�Ͻ�Ͳ��Ͻ�
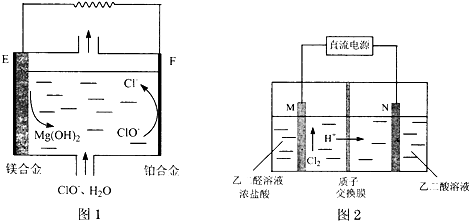
EΪ��ȼ�ϵ�صĸ��������������������F�缫�ϵĵ缫��ӦʽΪClO-+2e-+H2O�TCl-+2OH-��
��5����ȩ�ᣨHOOC-CHO�����л��ϳɵ���Ҫ�м��壮��ҵ���á�˫���ҳɶԵ缫�Ҿ��ɲ�����ȩ�ᣬ�����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ�ᣮ
����N�缫���Ҷ���������ȩ��ĵ缫��ӦʽΪHOOC-COOH+2e-+2H+�THOOC-CHO+H2O��
������2molH+ͨ�����ӽ���Ĥ��ȫ���뷴Ӧ�������ɵ���ȩ��Ϊ2mol��
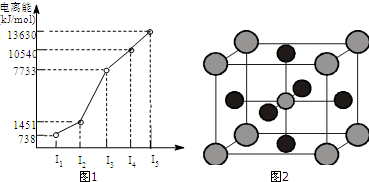
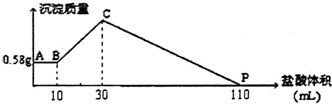 ����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ�У���0.58g��ɫ���������������õĻ���Һ�У�����1mol/L���ᣬ�����������������ɳ�����������ϵ��ͼ��ʾ����������NaOH����Ϊ��������
����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ�У���0.58g��ɫ���������������õĻ���Һ�У�����1mol/L���ᣬ�����������������ɳ�����������ϵ��ͼ��ʾ����������NaOH����Ϊ��������| A�� | 3.6g | B�� | 4g | C�� | 4.4g | D�� | 4.8g |
| A�� | ԭ��������a��b��c��d | |
| B�� | ���Ӱ뾶��A2+��B+��C2-��D- | |
| C�� | ���ʻ�ԭ�ԣ�B��A�����������ԣ�D��C | |
| D�� | ���ӻ�ԭ�ԣ�C2-��D-�����������ԣ�B+��A2+ |