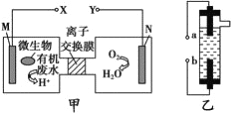
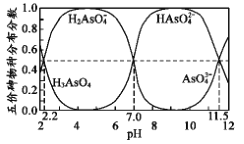
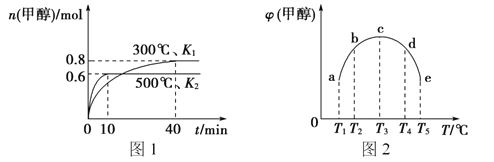
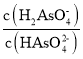��Ŀ����
����Ŀ��ijѧ����0.2000molL-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3mL��̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�������ش�
��1�����ϲ����д������___(����)��
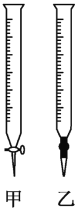
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��___(��ͼ��ѡ����������������)�С�
��3�����в���������ʵ����ƫ�����___(����)��
A.����ƿװҺǰ��������������ˮ
B.�ζ�ǰ���ζ��ܼ��������ݣ��ζ���������
C.����ʽ�ζ�����ȡҺ��ʱ���ͷ�Һ��ǰ�ζ���ǰ�������ݣ�֮����ʧ
��4���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע��___���жϵ���ζ��յ�������ǣ���ƿ����Һ___��
��5��������ʵ�����ݼ�¼����
�ζ����� | �������(mL) | NaOH��Һ�������(mL) | |
�ζ�ǰ | �ζ��� | ||
1 | 20.00 | 0.00 | 21.30 |
2 | 20.00 | 0.00 | 16.30 |
3 | 20.00 | 0.00 | 16.32 |
ͨ������ɵã�������Ũ��Ϊ___ molL-1(����������4λС��)��
���𰸡��٢� �� B ��ƿ����Һ��ɫ�ı仯 ��ƿ����Һǡ�ñ�Ϊdz��ɫ���Ұ�����ڲ���ɫ 0.1631molL-1
��������
��1�������к͵ζ��Ĺ淶�������жϸ������Ƿ���ȷ��
��2��������ҺӦװ�ڼ�ʽ�ζ����У�
��3�����ݹ�ϵ![]() �жϸ����ضԵζ������Ӱ�죻
�жϸ����ضԵζ������Ӱ�죻
��4���ζ�ʱ��Ӧʱ��ע����ƿ����Һ��ɫ�ı仯��ȷ�жϵζ��յ㣻
��5�����ݱ������ݣ����ù�ϵ![]() ���м��㡣
���м��㡣
��1����û����NaOH��Һ��ϴ��ʹNaOH��ҺŨ�Ƚ��ͣ��ٴ���
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壬����������ȷ��
�۵���Һ������0����0���̶������£������¶���������������ȷ��
�ܷ�̪��Һ������࣬3�μ��ɣ��ܴ���
���ñ�Һ�ζ����յ㣬���µζ���Һ����������һ�ζ��������Ϊ���ñ�Һ���������������ȷ��
���Դ�����Ǣ٢ܡ�
��Ϊ���٢ܣ�
��2��������ҺӦװ�ڼ�ʽ�ζ����У���Ϊ��ʽ�ζ��ܣ����ԣ�Ӧ����NaOH��Һע�����С�
��Ϊ���ң�
��3��A. ����ƿװҺǰ��������������ˮ�����ı����Һ���ʵ����ʵ�������Ӱ�죬Aѡ��������⣻
B. �ζ�ǰ���ζ��ܼ��������ݣ��ζ��������ݣ����ȡ�ı�Һ������ڵ��µı�Һ���������ʵ����ƫ��Bѡ��������⣻
C. ����ʽ�ζ�����ȡҺ��ʱ���ͷ�Һ��ǰ�ζ���ǰ�������ݣ�֮����ʧ����ʵ�ʷ�Ӧ�Ĵ���Һ�����С�ڶ�ȡ�Ĵ���Һ��������ĵı�Һ���ƫС������ʵ����ƫС��Cѡ��������⣻
��ѡB��
��4���ζ�ʱ���۾�Ӧע����ƿ����Һ��ɫ�ı仯��ȷ�жϵζ��յ㣻�����������꣬����ļ�ʹ��Һ��Ϊdz��ɫ�����������ڲ���ɫ����ζ��յ㣬���Ե���ζ��յ�������ǣ���ƿ����Һǡ�ñ�Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��Ϊ����ƿ����Һ��ɫ�ı仯����ƿ����Һǡ�ñ�Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��5�����ݱ������ݿ�֪����1�εζ�����NaOH��Һ���ƫ�����Ӧ��ȥ����2��3������NaOH��Һ���ƽ��Ϊ��(16.30mL+16.32mL)��2=16.31mL����c(HCl)= 0.2000molL-1��16.31mL��20.00mL=0.1631molL-1��
����0.1631molL-1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�