��Ŀ����
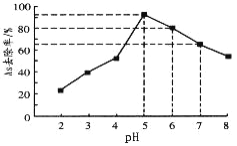
����Ŀ����ˮԼռ����������71%������ʮ�־�Ŀ���DZ������ͼ�ǿ�ˮ��Դ�ۺ����õĹ���ͼ������˵����ȷ( )

A. ���NaCl��Һʱ���������缫,�����ӷ���ʽΪ��2Cl- + 2H2O = 2OH-+ H2��+ Cl2��
B. ���±�м���Cl2���������û����嵥�ʣ��ù��������˻�ԭ�ԣ�Cl- > Br-
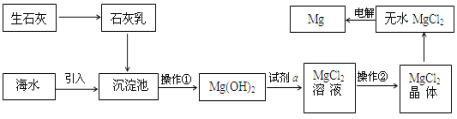
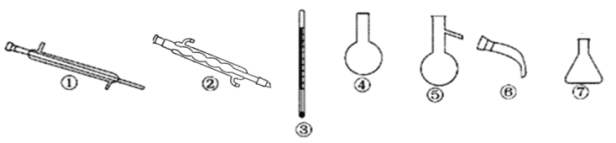
C. ʵ����ģ�⺣ˮ��ȡ��ˮ�����г�װ����ֻ�õ���������������ƿ���ƾ��ơ���ƿ
D. Br2 ��SO2��ˮ��Һ������Ӧ�����ӷ���ʽΪ��Br2 + SO2 + 2H2O =4H+ + 2Br- + SO42-
���𰸡�D
��������
A. �������ص���������ʧ��������Һ�е������Ӳ���ʧ�����������������ӷ���ʽ��д����A������
B. ���±�м���Cl2���������û����嵥�ʣ�֤�������������Դ�������B������
C. ʵ����ģ�⺣ˮ��ȡ��ˮ���õ���������������ƿ���ƾ��ơ���ƿ��������Ҫ���¶ȼ�����������ţ�ǹܵ���C������
D. Br2 ��SO2��ˮ��Һ������Ӧ��������������������ӷ���ʽΪ��Br2 + SO2 + 2H2O =4H+ + 2Br- + SO42-��D��ȷ��
����������������ǣ�D��

��ϰ��ϵ�д�
�����Ŀ