��Ŀ����
9��±������ȡ����Ӧ��ʵ���Ǵ�����ɵ�ԭ����ȡ����±�����е�±ԭ�ӣ���CH3Br+OH-����NaOH����CH3OH+Br-����NaBr�������л�ѧ����ʽ�в���ȷ���ǣ�������| A�� | CH3CH2Br+NaHS��CH3CH2SH+NaBr | |
| B�� | CH3I+CH3ONa��CH3OCH3+NaI | |
| C�� | CH3CH2Cl+CH3ONa��CH3CH2ONa+CH3Cl | |
| D�� | CH3CH2Cl+CH3CH2ONa����CH3CH2��2O+NaCl |
���� ����������Ϣ��±������ȡ����Ӧ��ʵ���Ǵ�����ɵ�ԭ����ȡ����±�����е�±ԭ�ӡ����ȸ��������Ӵ�������жϷ�Ӧ���д�����ɵ�ԭ���ţ�Ȼ������ɵ�ԭ����ȡ��±��ԭ�Ӽ��ɣ��ݴ���ɱ��⣮
��� �⣺A��NaHS����������Ӵ�����ɣ�ȡ���������е���ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Br+NaHS��CH3CH2HS+NaBr����A��ȷ��
B��CH3ONa�д�����ɵ�ԭ����ΪCH3O-��CH3O-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3I+CH3ONa��CH3OCH3+NaI����B��ȷ��
C��CH3ONa�д�����ɵ�ԭ����ΪCH3O-��CH3O-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Cl+CH3ONa��NaCl+CH3CH2OCH3����C����
D��CH3CH2ONa�д�����ɵ�ԭ����Ϊ��CH3CH2O-��CH3CH2O-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Cl+CH3CH2ONa����CH3CH2��2O+NaCl����D��ȷ��
��ѡC��
���� ���⿼����ȡ����Ӧ����Ŀ�ѶȲ���������Ǻ����������з�Ӧ��ԭ����������ض�ѧ������֪ʶ��ѵ���ͼ��飬����������ѧ����������������

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
19�����и��ֹ۵���ȷ���ǣ�������
| A�� | �ױ���ʹ���Ը��������Һ��ɫ��˵���ױ��к���̼̼˫�� | |
| B�� | ��ϩ�ܷ����ӳɷ�Ӧ�������ϩ����һ�������¾ۺ����ɸ߷��ӻ����� | |
| C�� | �л����ˮ��Һһ�����ܵ��� | |
| D�� | ͨ������³���̬�������е�̼ԭ��һ���������ĸ� |
20����ij�¶��£���20g����ϩ��C6H5CH=CH2������30g���У����û����Һ�У�̼Ԫ�ص����������ǣ�������
| A�� | 92.1% | B�� | 84.6% | C�� | 92.3% | D�� | 84.2% |
17�������йػ�ѧ����ʹ����ȷ���ǣ�������
| A�� | ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ��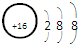 | |
| B�� | ԭ�Ӻ�����10�����ӵ���ԭ�ӣ�${\;}_{8}^{18}$O | |
| C�� | NH4Cl�ĵ���ʽ��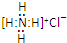 | |
| D�� | �����ᣨHClO4������Ԫ�صĻ��ϼ�Ϊ+5 |
4��ʵ�����Ʊ����״��ͱ�����Ļ�ѧԭ����ͼ1��ʾ����֪����ȩ�ױ��������������״��ķе�Ϊ205.3�棬��������۵�Ϊ121.7�棬�е�Ϊ249�棬�ܽ��Ϊ0.34g�����ѵķе�Ϊ34.8�棬������ˮ���Ʊ����״��ͱ��������Ҫ������ͼ2��ʾ���Ը���������Ϣ���ж�����˵��������ǣ�������


| A�� | ����������ȡ��Һ | |
| B�� | ������Һ�����ܽ����Ҫ�ɷ��DZ��״� | |
| C�� | �������������ò�Ʒ���DZ��״� | |
| D�� | ��������˵õ���Ʒ���DZ������ |
14�����б仯�����У�һ�������˻�ѧ�仯���ǣ�������
| A�� | ���� | B�� | ���� | C�� | ��ը | D�� | ȼ�� |
19������˵����ȷ���ǣ�������
| A�� | ��Ԫ����������Һ������ɫ | |
| B�� | ������Ϊ86��������Ϊ51���ԭ�ӿɱ�ʾΪ��$\stackrel{137}{86}$Rn | |
| C�� | CS2�ĵ���ʽ����$\underset{\stackrel{..}{S}}{..}$��$\underset{\stackrel{..}{C}}{..}$��$\underset{\stackrel{..}{S}}{..}$�� | |
| D�� | 6.0g���ᾧ���к���H+����ĿΪ0.1NA |

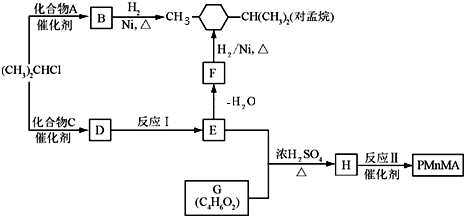

 ��
�� ��
�� ��
��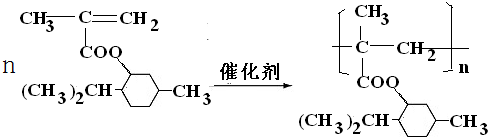 ��
��