��Ŀ����
1����������ʵ����ʵ�����ӷ���ʽ�У���ȷ���ǣ�������| A�� | ��Na2SO4��Һ�е���Ba��OH��2��Һ��������ɫ������Ba2++SO42-=BaSO4�� | |
| B�� | ��H2O�м�������Na2O2��������ɫ���壺2Na2O2+2H2O=O2��+4OH-+4Na+ | |
| C�� | ��Cl2ˮ�е�������Fe��NO3��2 ��Һ����Һ��Ϊ��ɫ��2Fe2++Cl2=2Fe3++2Cl- | |
| D�� | �����KI��Һ�еμ�����H2O2��Һ����Һ����ɫ��H2O2+2I-+2H+=I2+2H2O |
���� A��������������������Ӧ�������ᱵ������ˮ��©��������ˮ�ķ�Ӧ��
B������������ˮ��Ӧ�����������ƺ�������
C����ˮ��ʾ���ԣ�������������������ӵ���������ǿ������������������������ӷ�Ӧ��
D��˫��ˮ����ǿ�����ԣ��ܹ��������������ɵⵥ�ʣ�
��� �⣺A����Na2SO4��Һ�е���Ba��OH��2��Һ����Ӧ�������ᱵ��������Ӧ�����ӷ���ʽΪ��Ba2++SO42-=BaSO4������A��ȷ��
B����H2O�м�������Na2O2����Ӧ�����������ƺ���������Ӧ�����ӷ���ʽΪ��2Na2O2+2H2O=O2��+4OH-+4Na+����B��ȷ��
C����ˮ�����ԣ�������Һ����������ӵ���������ǿ������������������Ȳ��뷴Ӧ����ȷ�����ӷ���ʽΪ��6Fe2++8H++2NO3-=6Fe3++4H2O+2NO������C����
D�������KI��Һ�еμ�����H2O2��Һ�������ӱ�˫��ˮ�����ɵⵥ�ʣ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ��H2O2+2I-+2H+=I2+2H2O����D��ȷ��
��ѡC��
���� ���⿼�������ӷ���ʽ����д�жϣ���Ŀ�Ѷ��еȣ�ע���������ӷ���ʽ����дԭ����ȷ���ӷ���ʽ�����жϳ��÷�������鷴Ӧ��������Ƿ���ȷ���������ʲ���Ƿ���ȷ���������������ʵ���Ҫ������ѧʽ������Ƿ�����غ��ϵ���磺�����غ�͵���غ�ȣ���

 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�| A�� | H2O2+SO2��H2SO4 | |
| B�� | 3H2O2+2NaCrO2+2NaOH��2Na2CrO4+4H2O | |
| C�� | 2H2O2��2H2O+O2�� | |
| D�� | H2O2+2FeCl3��2FeCl2+2HCl+O2�� |
| A�� | ��Na2S2O3��Һ��ͨ������Cl2��S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+ | |
| B�� | ������Һ��ͨ��������CO2��CO2+2C6H5O-+H2O�T2C6H5OH+CO32- | |
| C�� | ������������ữ�ĸ��������Һ�У�5C2O42-+2MnO4-+16H+�T10CO2��+2Mn2++8H2O | |
| D�� | Ư����Һ�ڿ�����ʧЧ��ClO-+CO2+H2O�THClO+CO32- |
| A�� | ����ƿ����Ͳ�͵ζ����϶�����ʹ���¶ȣ���Ͳ������ƿ�ޡ�0���̶ȣ��ζ����С�0���̶ȣ�ʹ��ʱ�ζ���ˮϴ������ϴ��������ƿˮϴ������ϴ | |
| B�� | �õ�����ƽ������ѧҩƷʱ�������ȳ�С�ձ����������ٳ��������Լ����������������֮�ΪҩƷ������ | |
| C�� | �����Ż�ʱ������ϸɳ������𣻵����豸����Ļ��֣���������ĭ�������� | |
| D�� | ��4mL0.1mol•L-1��K2Cr2O7��Һ�еμ�����1mol•L-1��NaOH��Һ����Һ��ɫ�ӳ�ɫ��ɻ�ɫ |
 +
+ $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$
����˵���У�����ȷ���ǣ�������
| A�� | ������Ӧ��ԭ����������100% | |
| B�� | ���ǻ�������ĺ˴Ź���������6�����շ� | |
| C�� | ���ǻ���������Է����ӳɷ�Ӧ��ȡ����Ӧ����ȥ��Ӧ�����۷�Ӧ | |
| D�� | 1 mol���ǻ�������������NaOH��Һ��Ӧ������3 mol NaOH |
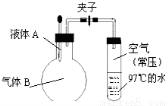
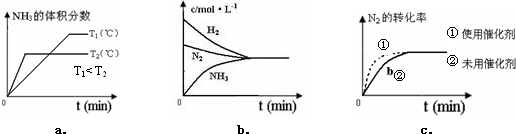
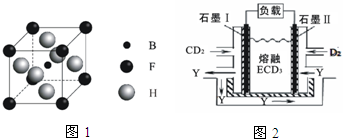 ��A��B��C��D��E��F��G��H����ԭ���������������Ԫ�أ�ԭ��������С��30����Aԭ�ӵĺ������������Ӳ�����ȣ�B�Ļ�̬ԭ����3����ͬ���ܼ��Ҹ��ܼ��е�������ȣ�D�Ļ�̬ԭ����B�Ļ�̬ԭ�ӵ�δ�ɶԵ�����Ŀ��ͬ��A��Eͬ���壬F�Ļ�̬ԭ��s�ܼ��ĵ���������p�ܼ��ĵ�������ȣ�B��Gͬ�壬H�Ļ�̬ԭ�ӵ�3d�����������4s��������4������ش��������⣺
��A��B��C��D��E��F��G��H����ԭ���������������Ԫ�أ�ԭ��������С��30����Aԭ�ӵĺ������������Ӳ�����ȣ�B�Ļ�̬ԭ����3����ͬ���ܼ��Ҹ��ܼ��е�������ȣ�D�Ļ�̬ԭ����B�Ļ�̬ԭ�ӵ�δ�ɶԵ�����Ŀ��ͬ��A��Eͬ���壬F�Ļ�̬ԭ��s�ܼ��ĵ���������p�ܼ��ĵ�������ȣ�B��Gͬ�壬H�Ļ�̬ԭ�ӵ�3d�����������4s��������4������ش��������⣺