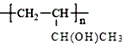题目内容
【题目】在实验室里,常用如图所示装置制取并收集氨气。请回答下列问题:
(1)原理与方法
①制取氨气的化学方程式为__________________________________;
②该收集氨气的方法为________。
A.向上排空气法 B.向下排空气法
(2)装置与操作
按下图组装仪器,进行实验。仪器a的名称为____________。

(3)思考与交流
①欲制取标准状况下4.48 L NH3,至少需要NH4Cl________g;
②实验室制取氨气,还可采用下图中的______(填“A”或“B”)。

【答案】2NH4Cl+Ca(OH)2![]() CaCl2+2NH3↑+2H2O B 酒精灯 10.7 A
CaCl2+2NH3↑+2H2O B 酒精灯 10.7 A
【解析】
(1)①实验室里用共热消石灰和氯化铵固体混合物制备氨气;
②NH3的密度比空气小;
(2)仪器a的名称为酒精灯;
(3)①依据化学方程式列式计算;
②实验室还可以将浓氨水滴入到生石灰中快速制备氨气。
(1)①实验室里用共热消石灰和氯化铵固体混合物制备氨气,反应的化学方程式为2NH4Cl+Ca(OH)2![]() CaCl2+2NH3↑+2H2O,故答案为:2NH4Cl+Ca(OH)2
CaCl2+2NH3↑+2H2O,故答案为:2NH4Cl+Ca(OH)2![]() CaCl2+2NH3↑+2H2O;
CaCl2+2NH3↑+2H2O;
②NH3的密度比空气小,所以用向下排空气法收集,故答案为:B;
(2)仪器a的名称为酒精灯,故答案为:酒精灯;
(3)①由方程式可得NH4Cl—NH3,标准状况4.48LNH3的物质的量为0.2mol,则NH4Cl的物质的量为0.2mol,NH4Cl的质量为0.2mol×53.5g/ mol=10.7g,故答案为:10.7;
②实验室还可以将浓氨水滴入到生石灰中快速制备氨气,故答案为:A。

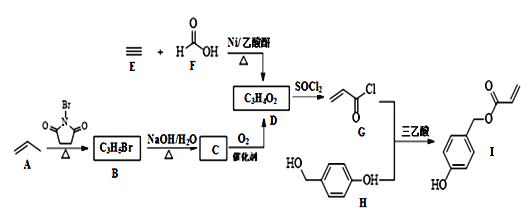
【题目】氯化亚砜(SOCl2)常用于医药、农药、染料工业,也可在有机合成工业中作氯化剂。已知:SOCl2的相关性质如下表所示:
颜色、状态 | 熔点 | 沸点 | 腐蚀性 | 水解 |
无色或微黄液体 | -105℃ | 78℃ | 强 | 极易水解 |
现利用如图装置制备SOCl2。
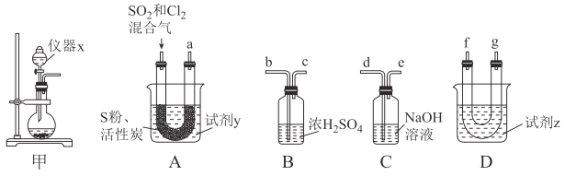
请回答下列问题:
Ⅰ.制备SO2和Cl2。
(1)本实验选用装置甲制备SO2和Cl2,装置甲中仪器x的名称为___;若以KMnO4和浓盐酸反应制备Cl2,反应的离子方程式为___。
Ⅱ.制备SOCl2。
以活性炭作为催化剂,SO2和C12可以和S粉在180~200℃时反应合成SOCl2,选用装置A、B、C、D进行制备(夹持、加热装置略去)。
(2)按气流从左到右的方向,装置A、B、C、D的连接顺序为___(填仪器接口的字母编号)。
(3)试剂y为___(填选项字母,下同);试剂z为___。
A.热水 B.乙醇 C.石蜡油 D.冰水
(4)装置A中U形管内发生反应的化学方程式为___。
(5)装置C的作用为___;若装置A处通入的SO2和Cl2的物质的量之比为1:3,则装置C中生成的盐为___(填化学式);请设计实验验证装置C中生成的盐中含有SO42-:____。