��Ŀ����
����Ŀ����1��15.6g Na2X�к�Na+ 0.4mol����Na2X��Ħ��������____________��
��2����NAΪ�����ӵ���������ֵ�����a g�����к��еķ�����Ϊb����c g�����ڱ�״���µ����Լ��_________________(�ú�NA��ʽ�ӱ�ʾ����
��3������£��ܶ�Ϊ1.25g/L��CO2��CH4��ɵĻ�������У�CO2���������Ϊ______��
��4�����и����뽺�������ص���______________________��
��±ˮ�㶹�� ��������ˮ �۾������ ����ˮ���� ��ѪҺ�� ���������γ� ������к� �����ʺ�ͺ�����¥ ���������
���𰸡�78g/mol ![]() L 42.86�� �ܢߢ�
L 42.86�� �ܢߢ�
��������
��1��������ʽṹ��֪Na2X�����ʵ���������n=![]() �ó����ۣ�
�ó����ۣ�
��2������cg�������з�����Ŀ���ٸ���n=![]() �����������ʵ���������V=nVm�������������
�����������ʵ���������V=nVm�������������
��3���ȸ���M=![]() �����������Ħ������������������CO2��CH4�����ʵ����������������Ϊx������������
�����������Ħ������������������CO2��CH4�����ʵ����������������Ϊx������������
��4�����ݽ����������Ӧ������
��1��Na2X�к�Na+ 0.4mol����n(Na2X)=0.2mol������n=![]() ��֪��M(Na2X)=
��֪��M(Na2X)=![]() =78g/mol���ʴ�Ϊ��78g/mol��
=78g/mol���ʴ�Ϊ��78g/mol��
��2��cg�������з�����ĿΪb��![]() ���������ʵ���Ϊ
���������ʵ���Ϊ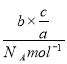 =
=![]() mol���ʱ�����������Ϊ
mol���ʱ�����������Ϊ![]() mol��22.4L/mol=
mol��22.4L/mol=![]() L���ʴ�Ϊ��
L���ʴ�Ϊ��![]() ��
��
��3������£��ܶ�Ϊ1.25g/L��CO2��CH4��ɵĻ�������Ħ������Ϊ1.25g/L![]() 22.4L/mol=28g/mol������������CO2��CH4�����ʵ����������������Ϊx��1��x����1mol������������������28g��Ҳ����44x+16(1��x) g����44x+16(1��x) = 28����ã�x=42.86�����ʴ�Ϊ��42.86����
22.4L/mol=28g/mol������������CO2��CH4�����ʵ����������������Ϊx��1��x����1mol������������������28g��Ҳ����44x+16(1��x) g����44x+16(1��x) = 28����ã�x=42.86�����ʴ�Ϊ��42.86����
��4���ٶ���Ϊ���壬������±��ʹ���巢���۳����뽺�������йأ�����������⣻
��������ˮ���õ�����������ˮ������������������������������壬������������ˮ�е����ʣ���ʹ���ˮ�г����������ﵽ��ˮ��Ŀ�ģ�����������⣻
���̳�Ϊ���壬���������֮һ�ǵ�Ӿ��ͨ����ѹֱ�����ȥ�������̳������Լ��ٶԿ�������Ⱦ���뽺��������йأ�����������⣻
����ˮ�������õ�������ˮ�������ݣ����÷�Һ�ķ������з���Ĺ��̣��뽺��������أ�����������⣻
��ѪҺ���ڽ��壬����������������������⣻
�����뺣�������γ������ޣ��糤�������ͻƺ��������γɣ����ں�ˮ�к����������壬��ˮ�뺣ˮ����ˮ������Ľ��������ʷ����۳����Ӷ��γ��������õ������ޣ��뽺������������һ����Ŀ�ĵ���йأ�����������⣻
������к����õ��������������������ӷ�Ӧ����ˮ�Ĺ��̣��뽺��������أ�����������⣻
�����Ҳ��һ�ֽ��壬��������¥���������ʺ�������һ�ֹ�ѧ����Ҳ�뽺��Ķ����ЧӦ�йأ�����������⣻
������������õ��dz�����ǿ�����ԣ��뽺���أ�����������⣻
�����������ܢߢ���������⣬
�ʴ�Ϊ���ܢߢᡣ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�