��Ŀ����
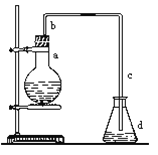
����Ŀ����ͼ��ʵ����������֤��ϩ��������Ӧ�����װ�ã���ش��������⣺

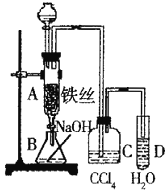
��1��������װ��������ȡ��ϩ�����а�����������ҩƷ�ǣ�
�����Ƭ ��ʯ����������̨ ��Բ����ƿ ���¶ȼƣ�����100�������¶ȼƣ�����200�����ƾ��� �ߵ����� ��˫�����ᵼ�ܡ���ѡ�õ������� �����ţ���
д������ϩ��ѧ����ʽ��_____________________________����Ӧ����һ��ʱ�������װ���л��Һ����ڣ������д̼�����ζ������������������� ����Ҫ��֤�и��������ɣ�Ӧ�����ɵ�����ͨ�� ��Һ��
��2��NaOH��Һ��������___________________����ʯ�ҵ������ǣ�_____________________��
��3��ʵ������У�������ˮ����ɫ�����ܵ�ԭ���ǣ�________ _____��
��4������ʲô�����˵�����������飿_______________________��
���𰸡���1���ܢ���CH3CH2OH![]() C2H4��+H2O��SO2��Ʒ����Һ
C2H4��+H2O��SO2��Ʒ����Һ
��2��������ϩ�����п��ܻ��е�SO2��CO2��������ϩ��
��3����ϩ����������ȫ��Ӧ
��4�����������Һ����ɫ������ʯ��ˮ�����
��������
���������
��1��ʵ�������Ҵ���Ũ����Ĵ������£�������170��ʱ��ȡ��ϩ�����������У������Ƭ ��ʯ����������̨ ��Բ����ƿ ���¶ȼƣ�����200�棩�ƾ��� ��˫�����ᵼ�ܣ�����Ҫ���Ǣ��¶ȼƣ�����100�棩���ߵ��������Ҵ���Ũ����Ĵ������£�������170��ʱ��ȡ��ϩ����ӦΪCH3CH2OH![]() C2H4��+H2O��ͬʱ����Ũ�������ˮ�ԣ�ʹ�����Ҵ�̼����ڣ�����Ũ�����ǿ�����ԣ������SO2���壬������Ʒ����Һ���顣
C2H4��+H2O��ͬʱ����Ũ�������ˮ�ԣ�ʹ�����Ҵ�̼����ڣ�����Ũ�����ǿ�����ԣ������SO2���壬������Ʒ����Һ���顣
��2��NaOH��Һ��������������ϩ�����п��ܻ��е�SO2��CO2����ʯ�ҵ�������������ϩ��
��3����ϩ����������ȫ��Ӧ��ʣ�����ϩʹ��ˮ��ɫ
��4�����黯ѧ�����ȶ������ܱ����Ը��������Һ������ȼ��ʱ����CO2����ʹ����ʯ��ˮ����ǡ�

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�