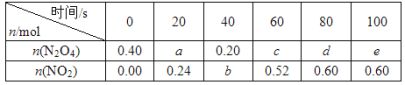
��Ŀ����
����Ŀ����ͭ����Ҫ�ɷ���Cu2S����ұ��ͭ����Ҫԭ�ϣ���ͭ����Ʒ�Ĵ��ȿ��������Ը��������Һ�ζ����ⶨ���÷�Ӧ�ɱ�ʾΪCu2S��![]() ��H����Cu2����
��H����Cu2����![]() ��Mn2����H2O��δ��ƽ��������˵���в���ȷ����
��Mn2����H2O��δ��ƽ��������˵���в���ȷ����
A. Cu2SΪ��ԭ���������� ![]() Ϊ����������ԭ
Ϊ����������ԭ
B. �������ͻ�ԭ�����ʵ���֮��Ϊ2��1
C. ��Ӧ��ÿ����1 mol Cu2S��ת��8 mol����
D. �ζ�ʱ���Բ���������ָʾ��
���𰸡�C
����������ƽ���ӷ���ʽΪCu2S��2MnO4-��8H+=2Cu2+��SO42-��2Mn2+��4H2O
A�Cu2S��ͭԪ�ػ��ϼ��ɣ�1����2�ۣ�����������Ԫ�ػ��ϼ���-2����6�ۣ�������������Cu2S����ԭ����MnO4-����Ԫ�ػ��ϼۣ�7����2�ۣ�����ԭ������MnO4-������������A��ȷ��B����ݷ���ʽ�������������������ͻ�ԭ�������ʵ���֮��2:1����B��ȷ��C���Ӧ��ÿ����1molCu2S��ת��10mol���ӣ���C������D���ΪMnO4-����������ɫ�����Եζ�ʱ���ü�����ָʾ������D��ȷ��

��ϰ��ϵ�д�
�����Ŀ