جâؤ؟ؤعبف
،¾جâؤ؟،؟رُ»¯ذ؟،¢ءٍ»¯ذ؟¶¼تاضطزھµؤ»ù´،»¯¹¤شءد،£
£¨1£©ZnO سë Al2O3 µؤ»¯ر§ذشضتدàثئ£¬ZnO شع NaOH بـز؛ضذ×ھ»¯³ة[Zn(OH)4]2µؤہë×س·½³جت½خھ_____________،£
£¨2£©»ً·¨ء¶ذ؟µأµ½µؤرُ»¯ذ؟ضذ؛¬سذا¦،¢حµبشسضت£¬جل´؟²½ضèبçدآ£؛
![]()
![]()
¢ظةدح¼ضذµؤ،°ہنؤخï،±خھ________£¨جر§ت½£©،£

¢عؤ³خآ¶بت±£¬شع·´س¦¢ٌµؤ·´س¦آ¯ضذ£¬ئًت¼ت± c(CO)خھ 0.3 molL1£¬·´س¦¹³جضذ CO2 µؤجه»·ضت ¦ص(CO2)بçح¼ثùت¾£¬شٍ·´س¦¢ٌµؤئ½؛â³£ت K£½_____،£
¢غدآءذ´ëت©سذہûسعجل¸ك·´س¦¢ٌضذ ZnO ×ھ»¯آتµؤتا________،£
a£®شِ´َ ZnO µؤح¶ءدء؟ b£®تتµ±¼سر¹ c£®½«ذ؟صôئّ¼°ت±·ضہë
¢ـ·´س¦¢ٍضذ£¬أ؟×ھزئ 1mol µç×س£¬·´س¦·إبب 174 kJ£¬شٍ H2£½_____________،£
£¨3£©²â¶¨رُ»¯ذ؟رùئ·´؟¶ب£؛³ئب، 0.5000g رùئ·£¬ثلبـ؛َ¶¨بفسع 250 mL بفء؟ئ؟ضذ£¬ز،شب،£ء؟ب، 25.00 mL ¸أبـز؛£¬سأ 0.04000 molL1 µؤ EDTA£¨Na2H2Y£©±ê×¼ز؛µخ¶¨ئنضذµؤ Zn2+£¨·´س¦·½³جت½خھ Zn2+£«H2Y2£½ZnY2£«2H+£¬شسضت²»·´س¦£©£¬ئ½ذذµخ¶¨ب´خ£¬ئ½¾ùدû؛ؤ EDTA ±ê×¼ز؛ 15.12mL،£
¢ظبôµخ¶¨¹ـخ´سأ EDTA ±ê×¼ز؛بَد´£¬²â¶¨½ل¹û½«___£¨جî،°ئ«¸ك،±،¢،°ئ«µح،±»ٍ،°²»±ن،±£©،£
¢عرùئ·´؟¶بخھ£؛________________£¨ءذ³ِ¼ئثمت½¼´؟ة£©،£
£¨4£©²تµçس«¹âئءضذµؤہ¶ة«س«¹â·غ؛¬سذ ZnS،£½«؛¬سذ 0.05mol ZnS µؤس«¹â·غبـسع 500mLرخثلضذ£¬حêب«بـ½â؛َ£¬بـز؛ضذ c(S2)،ـ__________ molL1،££¨زرضھ£؛Ksp(ZnS)£½2.5،ء1023£¬؛ِآشبـز؛جه»µؤ±ن»¯£©
،¾´ً°¸،؟ ZnO+2OH-+H2O=[Zn(OH)4]2- Zn 0.4mol/L c -696kJ/mol ئ«¸ك ![]() 2.5،ء10-22
2.5،ء10-22
،¾½âخِ،؟(1)ZnO؛حAl2O3µؤ»¯ر§ذشضتدàثئ£¬رُ»¯آءسëاâرُ»¯ؤئ·´س¦ةْ³ةئ«آءثلؤئ£¬ثùزشرُ»¯ذ؟سëاâرُ»¯ؤئµؤ·´س¦µؤ·½³جت½خھ£؛ZnO+H2O+2OH-=[Zn(OH)4]2-£¬¹ت´ً°¸خھ£؛ZnO+H2O+2OH-=[Zn(OH)4]2-£»
(2)¢ظ·´س¦¢ٌ£؛ZnO(s)+CO(g)Zn(g)+CO2(g)£¬ذ؟صôئّہنؤخھہنؤخہنؤخïخھ½ًتôذ؟£¬¹ت´ً°¸خھ£؛Zn£»
¢عؤ³خآ¶بت±£¬شع·´س¦¢ٌµؤ·´س¦آ¯ضذ£¬ئًت¼ت±c(CO)خھ0.3molL-1£¬·´س¦¹³جضذ´ïµ½ئ½؛âCO2µؤجه»·ضت¦ص(CO2)بçح¼ثùت¾خھ0.4£¬
ZnO(s)+CO(g)Zn(g)+CO2(g)
ئًت¼ء؟(mol/L) 0.3 0
±ن»¯ء؟(mol/L) x x
ئ½؛âء؟(mol/L) 0.3-x x
![]() =0.4£¬x=0.12£¬ئ½؛â³£تK=
=0.4£¬x=0.12£¬ئ½؛â³£تK=![]() =0.67£¬¹ت´ً°¸خھ£؛0.67£»
=0.67£¬¹ت´ً°¸خھ£؛0.67£»
¢غa£®شِ´َZnOµؤح¶ءدء؟£¬رُ»¯ذ؟خھ¹ججه²»س°دىئ½؛⣬رُ»¯ذ؟×ھ»¯آت²»±ن£¬¹تa´يخَ£»b£®·´س¦ا°؛َئّجهجه»²»±ن£¬تتµ±¼سر¹£¬²»س°دىئ½؛âزئ¶¯£¬¹تb´يخَ£»c£®½«ذ؟صôئّ¼°ت±·ضہ룬ئ½؛âصدٍ½ّذذرُ»¯ذ؟×ھ»¯آتشِ´َ£¬¹تcصب·£»¹ت´ً°¸خھ£؛c£»
¢ـ·´س¦¢ٍضذ2Zn(g)+O2(g)¨T2ZnO(s)£¬·´س¦ضذ2molZnحêب«·´س¦µç×س×ھزئ4mol£¬·´س¦أ؟×ھزئ1molµç×س£¬·´س¦·إبب174kJ£¬×ھزئ4molµç×س·´س¦·إبب696KJ£¬·´س¦ىت±ن،÷H=-696KJ/mol£¬¹ت´ً°¸خھ£؛-696KJ/mol£»
(3)¢ظµخ¶¨¹ـخ´سأEDTA±ê×¼ز؛بَد´£¬ؤع²مث®ؤ¤»لد،تح±ê×¼بـز؛£¬دû؛ؤ±ê×¼بـز؛جه»»لشِ´َ£¬²â¶¨½ل¹ûئ«¸ك£¬¹ت´ً°¸خھ£؛ئ«¸ك£»
¢ع³ئب،0.5000gرùئ·£¬ثلبـ؛َ¶¨بفسع250mLبفء؟ئ؟ضذ£¬ز،شب£®ء؟ب،25.00mL¸أبـز؛£¬سأ0.04000molL-1µؤEDTA(Na2H2Y)±ê×¼ز؛µخ¶¨ئنضذµؤZn2+(·´س¦·½³جت½خھZn2++H2Y2-¨TZnY2-+2H+£¬شسضت²»·´س¦)£¬ئ½ذذµخ¶¨ب´خ£¬ئ½¾ùدû؛ؤEDTA±ê×¼ز؛15.12mL£®
Zn2++H2Y2-¨TZnY2-+2H+£¬
1 1
n 15.12،ء10-3L،ء0.04000mol/L
n(ZnO)=n(Zn2+)=15.12،ء10-3L،ء0.04000mol/L£¬250mLبـز؛ضذn(ZnO)=15.12،ء10-3L،ء0.04000mol/L،ء![]() £¬رùئ·´؟¶ب=
£¬رùئ·´؟¶ب=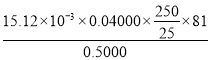 ،ء100%£¬¹ت´ً°¸خھ£؛
،ء100%£¬¹ت´ً°¸خھ£؛ 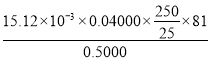 ،ء100%£»
،ء100%£»
(4)½«؛¬سذ0.05molZnSµؤس«¹â·غبـسع500mLرخثلضذ£¬حêب«بـ½â؛َبـز؛ضذذ؟ہë×سإ¨¶بc(Zn2+)=![]() =0.1mol/L£¬Ksp(ZnS)=2.5،ء10-23=c(Zn2+)c(S2-)£¬
=0.1mol/L£¬Ksp(ZnS)=2.5،ء10-23=c(Zn2+)c(S2-)£¬
c(S2-)،ـ2.5،ء10-22mol/L£¬¹ت´ً°¸خھ£؛2.5،ء10-22،£

 ±¸ص½ضذ؟¼؛®¼ظدµءذ´ً°¸
±¸ص½ضذ؟¼؛®¼ظدµءذ´ً°¸،¾جâؤ؟،؟£¨1£©زرضھH£«(aq)£«OH£(aq)=H2O(l) ¦¤H£½£57.3 kJmol1،£بôدٍب·فµبجه»،¢µبخïضتµؤء؟إ¨¶بµؤNaOHبـز؛ضذ·ض±ً¼سبë´×ثل،¢إ¨ءٍثل،¢د،دُثلضءا،؛أحêب«·´س¦£¬²¢½«ةدتِ¹³جضذ·إ³ِµؤببء؟·ض±ً¼اخھQ1 kJ،¢Q2 kJ،¢Q3 kJ،£شٍبصكµؤسةذ،µ½´َ¹طدµتا________(سأQ1،¢Q2،¢Q3±يت¾)،£
£¨2£©بçح¼ثùت¾Aخھإفؤثـءد°ه£¬ةدأوسذء½¸ِذ،؟×£¬·ض±ً²هبëخآ¶ب¼ئ؛ح»·ذخ²£ء§½ء°è°ô£¬ء½¸ِذ،؟ײ»ؤـ؟ھµأ¹´َ£¬ئنؤ؟µؤتا_____________________£» بôتµرéضذ²»¼س¸اإفؤثـءد°ه£¬شٍاَµأµؤضذ؛حببتضµ______£¨جîئ«´َ،¢ئ«ذ،،¢خقس°دى£©،£

£¨3£©تµرéتزسأ50 mL 0.50 molL1رخثل،¢50 mL 0.55 molL1 NaOHبـز؛ہûسأةدح¼×°ضأ£¬½ّذذ²â¶¨ضذ؛حببµؤتµرé،£¼ظةèرخثل؛حاâرُ»¯ؤئبـز؛µؤأـ¶ب¶¼تا1 g/cm3£¬سضضھضذ؛ح؛َةْ³ةبـز؛µؤ±ببببفc=4.18 J/(g،و)،£خھءث¼ئثمضذ؛حبب£¬تµرéت±»¹ذè²âء؟µؤت¾فسذ(جîذٍ؛إ)________،£
A£®·´س¦ا°رخثلµؤخآ¶ب B£®·´س¦ا°رخثلµؤضتء؟
C£®·´س¦ا°اâرُ»¯ؤئبـز؛µؤخآ¶ب D£®·´س¦ا°اâرُ»¯ؤئبـز؛µؤضتء؟
E£®·´س¦؛َ»ى؛دبـز؛µؤ×î¸كخآ¶ب F£®·´س¦؛َ»ى؛دبـز؛µؤضتء؟
£¨4£©ؤ³ر§ةْتµرé¼اآ¼ت¾فبçدآ£؛
تµرéذٍ؛إ | ئًت¼خآ¶بt1/،و | ضصض¹خآ¶بt2/،و | |
رخثل | اâرُ»¯ؤئبـز؛ | »ى؛دبـز؛ | |
1 | 20.0 | 20.1 | 23.4 |
2 | 20.2 | 20.4 | 23.6 |
3 | 20.5 | 20.6 | 23.8 |
زہ¾ف¸أر§ةْµؤتµرéت¾ف¼ئثم£¬¸أتµرé²âµأµؤضذ؛حبب¦¤Hخھ________،£(±£ءôبخ»سذذ§ت×ض)
