��Ŀ����
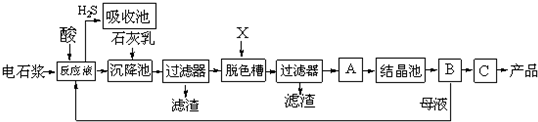
��������ƣ�Na2S2O3���׳Ʊ��շۣ�����������ҵ����Ӱ����Ҳ������ֽ��Ư�������ȼ��ȣ�ʵ���ҿ�ͨ�����·�Ӧ��ȡ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2��
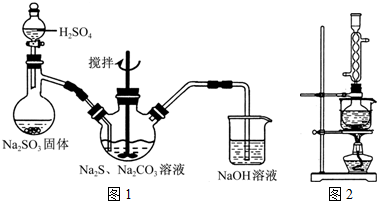
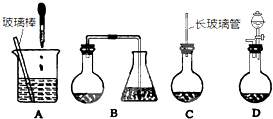
��1����ͼ1��ʾװ����ȡNa2S2O3������NaOH��Һ��������______���罫��Һ©���е�H2SO4�ij�Ũ���ᣬ��������ƿ�ڳ�Na2S2O3�����⣬����______���ѧʽ���������ɣ�
��2��Ϊ�ⶨ���ñ��շ���Ʒ��Na2S2O3?5H2O���������������ñ�����Һ���еζ�����Ӧ����ʽΪ2Na2S2O3+I2�T2NaI+Na2S4O6��
������KIO3��KI��HCl�����Ʊ�����Һ��д������ʱ��������Ӧ�����ӷ���ʽ��______��
��ȷ��ȡһ��������Na2S2O3?5H2O��Ʒ����ƿ�У���ˮ�ܽ⣬���μ�______��ָʾ�����������Ƶı�����Һ�ζ����ζ�ʱ���õIJ�����������ƿ�⣬����______��
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3?5H2O�����������IJ������______���ƫ�ߡ���ƫ�͡����䡱����
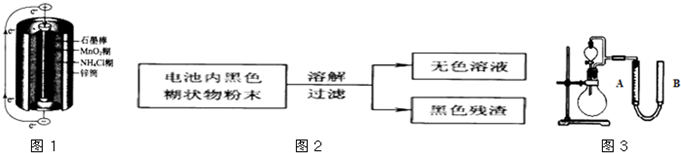
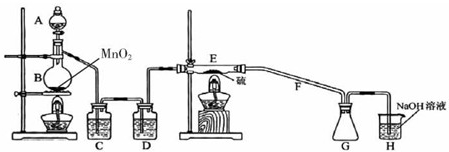
��3����ʵ���Na2S�Ĵ���Ҫ��ϸߣ�����ͼ2��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ����֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ����ᴿ��������Ϊ��
�ٽ��ѳ����õĹ�ҵNa2S����Բ����ƿ�У�������һ�������ľƾ�������ˮ��
�ڰ�ͼ2��ʾװ����������������������ͨ����ȴˮ��ˮԡ���ȣ�
�۴�______ʱ��ֹͣ���ȣ�����ƿȡ�£�
��______��
��______��
�����ù���ϴ�ӡ�����õ�Na2S?9H2O���壮

��1����ͼ1��ʾװ����ȡNa2S2O3������NaOH��Һ��������______���罫��Һ©���е�H2SO4�ij�Ũ���ᣬ��������ƿ�ڳ�Na2S2O3�����⣬����______���ѧʽ���������ɣ�
��2��Ϊ�ⶨ���ñ��շ���Ʒ��Na2S2O3?5H2O���������������ñ�����Һ���еζ�����Ӧ����ʽΪ2Na2S2O3+I2�T2NaI+Na2S4O6��
������KIO3��KI��HCl�����Ʊ�����Һ��д������ʱ��������Ӧ�����ӷ���ʽ��______��
��ȷ��ȡһ��������Na2S2O3?5H2O��Ʒ����ƿ�У���ˮ�ܽ⣬���μ�______��ָʾ�����������Ƶı�����Һ�ζ����ζ�ʱ���õIJ�����������ƿ�⣬����______��
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3?5H2O�����������IJ������______���ƫ�ߡ���ƫ�͡����䡱����
��3����ʵ���Na2S�Ĵ���Ҫ��ϸߣ�����ͼ2��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ����֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ����ᴿ��������Ϊ��
�ٽ��ѳ����õĹ�ҵNa2S����Բ����ƿ�У�������һ�������ľƾ�������ˮ��
�ڰ�ͼ2��ʾװ����������������������ͨ����ȴˮ��ˮԡ���ȣ�
�۴�______ʱ��ֹͣ���ȣ�����ƿȡ�£�
��______��
��______��
�����ù���ϴ�ӡ�����õ�Na2S?9H2O���壮
��1��װ������������������β������Ҫ���ã���Ϊԭ����������������Ⱦ�����岻���ŷŵ������У��Է�ֹ��Ⱦ���������� ������з�Ӧ������ �����������������Ȼ��ƣ�
�ʴ�Ϊ�����ն��������β������ֹ��Ⱦ������NaCl��
��2������KIO3��KI��HCl�����Ʊ�����Һ������KIO3��KI������Һ�з�����������ԭ��Ӧ���ɵ��ʵ⣬��Ӧ�����ӷ���ʽΪ��IO3-+5I-+6H+=3I2+3H2O��
�ʴ�Ϊ��IO3-+5I-+6H+=3I2+3H2O��
�������Ƶĵ���Һ�ζ���������ƣ����õⵥ�����ⵥ�ʱ���ָʾ��Ӧ�յ㣻����Һ���������ԣ�Ӧѡ����ʽ�ζ��ܣ�
�ʴ�Ϊ��������Һ����ʽ�ζ��ܣ�
�۵ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ı���Һ�����С�����ʹ��Ʒ��Na2S2O3?5H2O�����������IJ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��3�����ᴿ���Ƶ�ʵ�鲽��Ͳ������̿�֪��
������Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ��������ᴿ���ƾ����ʵ�鲽��ͷ�����֪������ƿ�й��岻�ټ��٣�˵������ȫ���ܽ��ھƾ��У����ܵ����ʳ������˳�ȥ���ʴ�Ϊ����ƿ�й��岻�ټ��٣�
���¶����������ھƾ����ܽ����������Һ���˵õ���Һ����Ҫ�����Ƶľƾ���Һ���ʴ�Ϊ�����ȹ��ˣ�
���ȵ���Һͨ����ȴ���ᾧ�����˵õ����壬�ʴ�Ϊ����������Һ��ȴ�ᾧ�����˵õ����ƽᾧˮ���
�ʴ�Ϊ�����ն��������β������ֹ��Ⱦ������NaCl��
��2������KIO3��KI��HCl�����Ʊ�����Һ������KIO3��KI������Һ�з�����������ԭ��Ӧ���ɵ��ʵ⣬��Ӧ�����ӷ���ʽΪ��IO3-+5I-+6H+=3I2+3H2O��
�ʴ�Ϊ��IO3-+5I-+6H+=3I2+3H2O��
�������Ƶĵ���Һ�ζ���������ƣ����õⵥ�����ⵥ�ʱ���ָʾ��Ӧ�յ㣻����Һ���������ԣ�Ӧѡ����ʽ�ζ��ܣ�
�ʴ�Ϊ��������Һ����ʽ�ζ��ܣ�
�۵ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ı���Һ�����С�����ʹ��Ʒ��Na2S2O3?5H2O�����������IJ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��3�����ᴿ���Ƶ�ʵ�鲽��Ͳ������̿�֪��
������Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ��������ᴿ���ƾ����ʵ�鲽��ͷ�����֪������ƿ�й��岻�ټ��٣�˵������ȫ���ܽ��ھƾ��У����ܵ����ʳ������˳�ȥ���ʴ�Ϊ����ƿ�й��岻�ټ��٣�
���¶����������ھƾ����ܽ����������Һ���˵õ���Һ����Ҫ�����Ƶľƾ���Һ���ʴ�Ϊ�����ȹ��ˣ�
���ȵ���Һͨ����ȴ���ᾧ�����˵õ����壬�ʴ�Ϊ����������Һ��ȴ�ᾧ�����˵õ����ƽᾧˮ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�