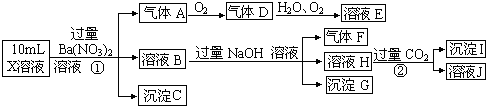
��Ŀ����
2������˵����ȷ���ǣ�������| A�� | ���ά�������������������մɡ��������������ǽ������ϣ�PLA��PE������ȩ��֬�����˹��ϳɸ߷��Ӳ��� | |
| B�� | ������������Ǽ���������������ͨ����ѧ��Ӧ�������� | |
| C�� | ��ѧ�ҷ���һ���µ�CO2���壬��CO2������м�ǿ��Ӳ�ȣ�����CO2���ӹ��ɵĿռ�������״�ṹ | |
| D�� | ���µ��ȼҵ�������ӽ���Ĥ���۵�ⱥ��ʳ��ˮ�������������������ռ�ģ������е����ӽ���Ĥ�ȿ����������ӽ���ĤҲ�����������ӽ���Ĥ |
���� A���������մɲ������ǽ������ϣ�
B����������ǽ����������γɻ����
C��CO2�Ƿ��Ӿ��壻
D����ⱥ��ʳ��ˮ������ʱ��Cl-�������ŵ磬H+�������ŵ磮
��� �⣺A���������к��н���Ԫ�أ����������մɲ������ǽ������ϣ���A����
B����������ǽ����������γɻ�����Ӷ��ﵽ����������Ŀ�ģ����Ǽ�������������B��ȷ��
C��CO2�Ƿ��Ӿ��壬���Ӽ���ѧ������������̼���Ӽ䲻���γɿռ���״�ṹ����C����
D����ⱥ��ʳ��ˮ������ʱ��Cl-�������ŵ磺2Cl--2e-=Cl2����������ˮ��H+�������ŵ磺2H++2e-=H2����OH-���������ɣ������м�Ӧ���������ӽ���Ĥ���Ӷ�ʹNaOH���������ɣ�������������ӽ���Ĥ����NaOH�����������ɣ����������ɵ������������������ݳ�����D����
��ѡB��
���� ���⿼�����ȼҵ���������������ԭ���ȣ�Ӧע��������ȼҵ�У������м�Ӧ���������ӽ���Ĥ��

��ϰ��ϵ�д�
�����Ŀ
19�����и��������У�����Ϊͬ���칹����ǣ�������
| A�� | ˮ��� | B�� | CH4��CH3CH3 | C�� | 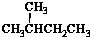 �� ��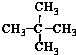 | D�� | 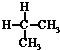 �� ��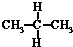 |
10����Ӧ2SO2+O2?2SO3���ܱ������н��У����й��ڸ÷�Ӧ��˵��������ǣ�������
| A�� | �����¶��ܼӿ췴Ӧ���� | B�� | ʹ��ǡ���Ĵ����ܼӿ췴Ӧ���� | ||
| C�� | ����O2��Ũ���ܼӿ췴Ӧ���� | D�� | SO2��O2��100%ת��ΪSO3 |
17�����н���ʵ����ʵ�����ӷ���ʽ��ȷ���ǣ�������
| A�� | ϡ����������������Һ��ϲ���������Ba2++SO${\;}_{4}^{2-}$=BaSO4�� | |
| B�� | �ô���ʯ��ϡ�����Ʊ�������̼��CO${\;}_{3}^{2-}$+2H+=CO2��+H2O | |
| C�� | ������������Һ����������Cl2+2OH-=ClO-+Cl-+H2O | |
| D�� | ͭƬ����ϡ�����в������壺Cu+4H++2NO${\;}_{3}^{-}$=Cu2++2NO2��+2H2O |
7���±���A��B��C��D�����л�����й���Ϣ��
��ش��������⣮
��1��д���л���C�Ľṹ��ʽHCOOH��
��2��д��B��D�Ļ�ѧ��Ӧ����ʽCH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
��3��д��A��NH3��һ�������·�Ӧ���ɱ�ϩ�����Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�CH2=CHCOOH+NH3��CH2=CHCOONH2+H2O����Ӧ����ȡ����Ӧ��
��4����ϩ�����ж���ͬ���칹�壬��д��������ͬʱ����ȩ����̼̼˫����3��ͬ���칹��Ľṹ��ʽCH2=CHNHCHO��CH2=C��NH2��CHO��CH��NH2��=CHCHO��
| A | 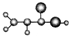 ����C��H��O����Ԫ����� �����ģ��Ϊ�� ������NH3��һ�������·�Ӧ���ɱ�ϩ����CH2=CHCONH2 ����Է�������Ϊ72 |
| B | ����C��H��O����Ԫ����ɡ�������Na��Ӧ����������NaOH��Һ��Ӧ ������A��Ӧ������Է�������Ϊ100���� |
| C | ����Է���������B��ͬ�����ܱ����Ƶ�������ͭ����Һ���� ������NaHCO3��Һ��Ӧ�ų�CO2���� |
| D | ����ʹ������Ȼ�̼��Һ��ɫ��������ˮ��һ�������·�Ӧ����B |
��1��д���л���C�Ľṹ��ʽHCOOH��
��2��д��B��D�Ļ�ѧ��Ӧ����ʽCH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
��3��д��A��NH3��һ�������·�Ӧ���ɱ�ϩ�����Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�CH2=CHCOOH+NH3��CH2=CHCOONH2+H2O����Ӧ����ȡ����Ӧ��
��4����ϩ�����ж���ͬ���칹�壬��д��������ͬʱ����ȩ����̼̼˫����3��ͬ���칹��Ľṹ��ʽCH2=CHNHCHO��CH2=C��NH2��CHO��CH��NH2��=CHCHO��
14����v��������v���棩�ֱ��ʾ���淴Ӧ������Ӧ���ʺ��淴Ӧ���ʣ���һ���¶��¿��淴ӦN2+3H2$\frac{\underline{\;���¡���ѹ\;}}{����}$2NH3�ﵽƽ��ʱ��������
| A�� | V���棩��V������ | B�� | V���棩��V������ | ||
| C�� | V���棩��V�����������淴Ӧֹͣ | D�� | V���棩=V�����������淴Ӧ�Խ��� |
11��������Һ���������ʵ���Ũ�ȹ�ϵһ����ȷ���ǣ�������
| A�� | 25��ʱpH=10��NaOH��Һ��pH=10�İ�ˮ�У�c��Na+����c��NH${\;}_{4}^{+}$�� | |
| B�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ�c��CH3COO-��+c��OH-��=c��H+��+c��CH3COOH�� | |
| C�� | ��NaHA��Һ�У�H2AΪ���ᣩ��c��Na+����c��HA-����c��OH-����c��H+�� | |
| D�� | �����£���0.01 mol•L-1 NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ�c��Na+����c��SO${\;}_{4}^{2-}$����c��NH${\;}_{4}^{+}$����c��OH-��=c��H+�� |
 �л���A��ʯ���ѽ����Ҫ����֮һ�����״���µ��ܶ�Ϊ1.25g/L����Ӧ���ܻ���Ŀǰһ����Ҫ�Ļ������⣬���ɵľۺ���G��C3H4O3��n��һ�ֿɽ������ϣ��й�ת����ϵ��ͼ��ʾ��
�л���A��ʯ���ѽ����Ҫ����֮һ�����״���µ��ܶ�Ϊ1.25g/L����Ӧ���ܻ���Ŀǰһ����Ҫ�Ļ������⣬���ɵľۺ���G��C3H4O3��n��һ�ֿɽ������ϣ��й�ת����ϵ��ͼ��ʾ�� +CaCl2+2H2O��
+CaCl2+2H2O��