��Ŀ����
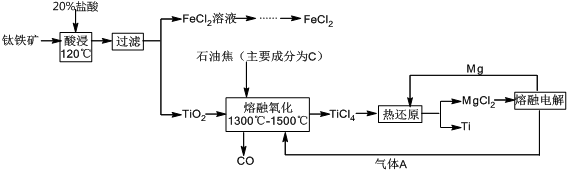
 �ϳɰ����Ʊ�������������Ҫ�Ļ�������������ij���������ϳɰ����Ʊ������������������
�ϳɰ����Ʊ�������������Ҫ�Ļ�������������ij���������ϳɰ����Ʊ��������������������1����ij�¶������Ϊ200L�İ��ϳ����У��Է�Ϊ��λ��ʱ����ϲ�ø����ʵ�Ũ�ȣ�mol?L-1�����±���
| 0min | l min | 2min | 3min | 4min | |
| N2 | 1.500 | 1.400 | 1.200 | c1 | c1 |
| H2 | 4.500 | 4.200 | 3.600 | c2 | c2 |
| NH3 | 0 | 0.200 | 0.600 | c3 | c3 |
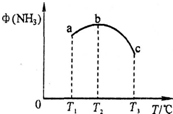
��2����Ժϳɰ���Ӧ���������о������ݻ���Ϊ10L��a��b��c������ͬ�ܱ������зֱ����1mol N2��3mol H2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3��������������ͬ����£�ʵ���÷�Ӧ�����е�5minʱ��NH3�����������ͼ��ʾ��������˵����ȷ����
A��a��b��C������5minʱ������Ӧ���ʴ�СΪ��b��a��c
B���ﵽƽ��ʱ��a��b��c��N2ת����Ϊa��b��c
C��5minʱ��a��b��c�������еķ�Ӧ�����ܴﵽƽ��״̬
D��������b�е�ƽ��״̬ת�䵽����c�е�ƽ��״̬���ɲ�ȡ�Ĵ�ʩ�����»��ѹ
��3����֪��N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol
4NH3��g��+5O2��g���T4NO��g��+6H2O��g������H=-905kJ/mol
2H2��g��+O2��g���T2H2O��g������H=-483.6kJ/mol
���ڸ������£����ϳ�������������Ӧ���Ȼ�ѧ����ʽΪ��
��4������Cu�����백ˮ����˫��ˮ������Ӧ����������ߵĻ����Һ��Ӧ��������ɫ��Һ��д���÷�Ӧ�����ӷ���ʽ��
��5��ȡ200mL������ǡ����32g Cu2S��ȫ��Ӧ����֪���ᱻ��ԭ�ɵ����ʵ�����NO��NO2�������CuSO4��Cu��NO3��2���ɣ������ù�ҵ�����Ũ����
���㣺����ٷֺ������¶ȡ�ѹǿ�仯����,��ѧ����ʽ���йؼ���,�Ȼ�ѧ����ʽ,��Ӧ���ʵĶ�����ʾ����,��ѧƽ���Ӱ������
ר�⣺������,��ѧƽ��ר��
��������1������v=
����v��N2�������ݵ�����ת���ʼ���������ת���ʣ�
��2������ͼ���Ӱ�컯ѧƽ������ؽ����жϣ�a�з�Ӧδ��ƽ�⣬b��c�еķ�Ӧ�ﵽƽ��״̬��
��3�����ø�˹���ɽ��м��㣻
��4��ͭ��˫��ˮ�Ͱ�ˮ��Ӧ�����������ӷ���ʽΪ��Cu+4NH3?H2O+H2O2=[Cu��NH3��4]2++4H2O+2OH-��
��5�����ݵ�ʧ�����غ��Ԫ���غ���м��㣮
| ��c |
| t |
��2������ͼ���Ӱ�컯ѧƽ������ؽ����жϣ�a�з�Ӧδ��ƽ�⣬b��c�еķ�Ӧ�ﵽƽ��״̬��
��3�����ø�˹���ɽ��м��㣻
��4��ͭ��˫��ˮ�Ͱ�ˮ��Ӧ�����������ӷ���ʽΪ��Cu+4NH3?H2O+H2O2=[Cu��NH3��4]2++4H2O+2OH-��
��5�����ݵ�ʧ�����غ��Ԫ���غ���м��㣮
���
�⣺��1���ɱ���֪��������ʼŨ��Ϊ1.5mol/L��2min������Ũ��Ϊ1.2mol/L��
����2Сʱ�ڵ���������Ϊv��N2��=
=0.15mol?L-1?min-1��
��֪N2��ת����Ϊa����������ת������������ת������ȣ���H2��ת����Ϊa��
�ʴ�Ϊ��0.15mol?L-1?min-1��a��
��2��A�������¶ȣ���ѧ��Ӧ���ʼӿ죬��a��b��C������5minʱ������Ӧ���ʴ�СΪ��c��b��a����A����
B���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ����ʴﵽƽ��ʱ��a��b��c��N2ת����Ϊa��b��c����B��ȷ��
C���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����5minʱ��a�з�Ӧδ��ƽ�⣬b��c�еķ�Ӧ�ﵽƽ��״̬����C����
D���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����Ӧ���������С�ڷ�Ӧǰ����Сѹǿ��ƽ�������ƶ����ʽ�����b�е�ƽ��״̬ת�䵽����c�е�ƽ��״̬���ɲ�ȡ�Ĵ�ʩ�����»��ѹ����D��ȷ��
�ʴ�Ϊ��BD��
��3����N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol
��4NH3��g��+5O2��g���T4NO��g��+6H2O��g������H=-905kJ/mol
��2H2��g��+O2��g���T2H2O��g������H=-483.6kJ/mol��
��-
��+
�۵�N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��
��4��ͭ��˫��ˮ�Ͱ�ˮ��Ӧ�����������ӷ���ʽΪ��Cu+4NH3?H2O+H2O2=[Cu��NH3��4]2++4H2O+2OH-��
�ʴ�Ϊ��Cu+4NH3?H2O+H2O2=[Cu��NH3��4]2++4H2O+2OH-��
��5��32g Cu2S�����ʵ���Ϊ��
=0.2mol��ͭ�Ļ��ϼ���+1������Ϊ+2�ۣ���ת�Ƶĵ�����Ϊ��0.2��2=0.4mol����Ļ��ϼ���-2������Ϊ+6�ۣ���ת�Ƶĵ�����Ϊ��0.2��8=1.6mol���ʹ�ת�Ƶĵ�����Ϊ0.4+1.6=2mol��
Cu2S��0.2mol���������غ��֪��CuSO4��0.2mol������ͭ�غ��֪��Cu��NO3��2��0.2mol��n��NO3-��=0.4mol��
���ᱻ��ԭ�ɵ����ʵ�����NO��NO2����NO��NO2�����ʵ���Ϊx����ת�Ƶĵ�����Ϊ��3x+x=4x=2��x=0.5mol������������ʵ���Ϊ��0.5+0.5+0.4=1.4mol��c��HNO3��=
=7mol?L-1��
�ʴ�Ϊ��7��
����2Сʱ�ڵ���������Ϊv��N2��=
| 1.5mol/L-1.2mol/L |
| 2min |
��֪N2��ת����Ϊa����������ת������������ת������ȣ���H2��ת����Ϊa��
�ʴ�Ϊ��0.15mol?L-1?min-1��a��
��2��A�������¶ȣ���ѧ��Ӧ���ʼӿ죬��a��b��C������5minʱ������Ӧ���ʴ�СΪ��c��b��a����A����
B���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ����ʴﵽƽ��ʱ��a��b��c��N2ת����Ϊa��b��c����B��ȷ��
C���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����5minʱ��a�з�Ӧδ��ƽ�⣬b��c�еķ�Ӧ�ﵽƽ��״̬����C����
D���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����Ӧ���������С�ڷ�Ӧǰ����Сѹǿ��ƽ�������ƶ����ʽ�����b�е�ƽ��״̬ת�䵽����c�е�ƽ��״̬���ɲ�ȡ�Ĵ�ʩ�����»��ѹ����D��ȷ��
�ʴ�Ϊ��BD��
��3����N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol
��4NH3��g��+5O2��g���T4NO��g��+6H2O��g������H=-905kJ/mol
��2H2��g��+O2��g���T2H2O��g������H=-483.6kJ/mol��
��-
| 1 |
| 2 |
| 3 |
| 2 |
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��
��4��ͭ��˫��ˮ�Ͱ�ˮ��Ӧ�����������ӷ���ʽΪ��Cu+4NH3?H2O+H2O2=[Cu��NH3��4]2++4H2O+2OH-��
�ʴ�Ϊ��Cu+4NH3?H2O+H2O2=[Cu��NH3��4]2++4H2O+2OH-��
��5��32g Cu2S�����ʵ���Ϊ��
| 32 |
| 160 |
Cu2S��0.2mol���������غ��֪��CuSO4��0.2mol������ͭ�غ��֪��Cu��NO3��2��0.2mol��n��NO3-��=0.4mol��
���ᱻ��ԭ�ɵ����ʵ�����NO��NO2����NO��NO2�����ʵ���Ϊx����ת�Ƶĵ�����Ϊ��3x+x=4x=2��x=0.5mol������������ʵ���Ϊ��0.5+0.5+0.4=1.4mol��c��HNO3��=
| 1.4 |
| 0.2 |
�ʴ�Ϊ��7��
���������⿼�黯ѧ��Ӧ���ʼ���ѧƽ����㡢�Ȼ�ѧ����ʽ����д��������ԭ��Ӧ����ؼ���ȣ������ܴ��ѶȽϴ�

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ
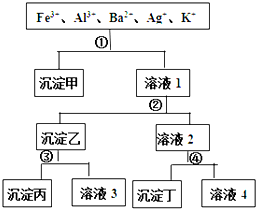 ij��Һ����Fe3+��Al3+��Ba2+��Ag+��K+���������ӣ����ù�����H2SO4��NaOH��NH3?H2O��NaCl������Һ������ͼ��ʾ�IJ���ֿ��������ӣ������ж���ȷ���ǣ�������
ij��Һ����Fe3+��Al3+��Ba2+��Ag+��K+���������ӣ����ù�����H2SO4��NaOH��NH3?H2O��NaCl������Һ������ͼ��ʾ�IJ���ֿ��������ӣ������ж���ȷ���ǣ�������| A�����������������������������Ļ���� |
| B����Һ3�к���Al3+ |
| C����Һ4�������������ӣ��ֱ���H+��Na+��K+ |
| D���Լ�����NaCl���Լ�����H2SO4 |
��֪ij���ӵ������������ȷ���ģ�������
| A�������� | B������������ |
| C���˵���� | D�����ԭ������ |