��Ŀ����
(15��)I��NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1����ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(NH4+) (����ڡ��������ڡ���С�ڡ�)0.1 mol��L��1NH4HSO4��c(NH4+)��
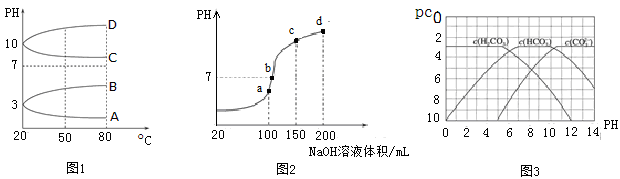
��2����ͼ1��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯�������� (��д��ĸ)��
��20��ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)�� mol��L��1(����ֵ)��
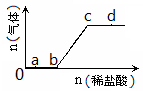
��3������ʱ����100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ��
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������� ����b�㣬��Һ�и�����Ũ���ɴ�С������˳���� ��
II��pC��ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ, ����pH����ij��Һ���ʵ�Ũ��Ϊ1��10��3 mol��L��1�������Һ�и����ʵ�pC����lg10��3��3����֪H2CO3��Һ�д�������ƽ�⣺CO2��H2O H2CO3��H2CO3
H2CO3��H2CO3 H����HCO3-��HCO3-
H����HCO3-��HCO3- H����CO32-
H����CO32-
ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺
��1����pH��9ʱ��H2CO3��Һ��Ũ�����ĺ�̼Ԫ�ص�����Ϊ ��
��2��pH<4ʱ����Һ��H2CO3��pC����Լ����3��ԭ���� ��
��3����֪M2CO3Ϊ���������Ksp�ı���ʽΪ ��������ij��Һ�е�M����̼����(Ksp��)����ʽ������ȫ���������Һ�е�CO32-��pC���ֵΪ (��Һ�е�����Ũ��С��1��10��5 mol��L��1ʱ��������ȫ)��

��1����ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(NH4+) (����ڡ��������ڡ���С�ڡ�)0.1 mol��L��1NH4HSO4��c(NH4+)��
��2����ͼ1��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯�������� (��д��ĸ)��
��20��ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)�� mol��L��1(����ֵ)��
��3������ʱ����100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ��
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������� ����b�㣬��Һ�и�����Ũ���ɴ�С������˳���� ��
II��pC��ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ, ����pH����ij��Һ���ʵ�Ũ��Ϊ1��10��3 mol��L��1�������Һ�и����ʵ�pC����lg10��3��3����֪H2CO3��Һ�д�������ƽ�⣺CO2��H2O
 H2CO3��H2CO3
H2CO3��H2CO3 H����HCO3-��HCO3-
H����HCO3-��HCO3- H����CO32-
H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺
��1����pH��9ʱ��H2CO3��Һ��Ũ�����ĺ�̼Ԫ�ص�����Ϊ ��
��2��pH<4ʱ����Һ��H2CO3��pC����Լ����3��ԭ���� ��
��3����֪M2CO3Ϊ���������Ksp�ı���ʽΪ ��������ij��Һ�е�M����̼����(Ksp��)����ʽ������ȫ���������Һ�е�CO32-��pC���ֵΪ (��Һ�е�����Ũ��С��1��10��5 mol��L��1ʱ��������ȫ)��
��1����(1��)
��2����A (1��) ��10��3��10-11mol��L��1(2��,д��10��3 mol��L��1Ҳ����)
��3��a(2��) c(Na��)>c(SO42-)>c(NH4+)>c(OH��)��c(H��)(2��)
II����1��HCO3- (1��)
��2��c(H��)�����H2CO3 H����HCO3-ƽ�������ƶ��ų�CO2��̼��Ũ�ȱ��ֲ��䣻
H����HCO3-ƽ�������ƶ��ų�CO2��̼��Ũ�ȱ��ֲ��䣻
��3��Ksp��c2(M��)��c(CO32-)(2��) 2(2��)
��2����A (1��) ��10��3��10-11mol��L��1(2��,д��10��3 mol��L��1Ҳ����)
��3��a(2��) c(Na��)>c(SO42-)>c(NH4+)>c(OH��)��c(H��)(2��)
II����1��HCO3- (1��)
��2��c(H��)�����H2CO3
 H����HCO3-ƽ�������ƶ��ų�CO2��̼��Ũ�ȱ��ֲ��䣻
H����HCO3-ƽ�������ƶ��ų�CO2��̼��Ũ�ȱ��ֲ��䣻��3��Ksp��c2(M��)��c(CO32-)(2��) 2(2��)
�����������1����ͬ�����£���Ϊ NH4++H2O
 NH3��HO+H+����������NH4+��ˮ�⣬0.1 mol��L��1 NH4Al(SO4)2��c(NH4+)С��0.1 mol��L��1NH4HSO4��c(NH4+)��
NH3��HO+H+����������NH4+��ˮ�⣬0.1 mol��L��1 NH4Al(SO4)2��c(NH4+)С��0.1 mol��L��1NH4HSO4��c(NH4+)����2��������ˮ��NH4Al(SO4)2��ˮ�⣬pH��7�������¶ȣ�ˮ��̶����pH��С���ʱ仯��������A��
��20��ʱ��0.1 mol��L��1NH4Al(SO4)2��ҺpH=3��c(H��)=10��3mol��L��1���ɵ���غ��֪��
2c(SO42-)+c(OH��)=c(NH4+)+3c(Al3��)+c(H��)��2c(SO42-)��c(NH4+)��3c(Al3��)��c(H��) ��c(OH��)=(10��3��10-11)mol��L��1��
��3����ͼ����ˮ�ж�ˮ�ĵ������������ã�������ʱ������NH4+��ˮ�⣬��ˮ�ĵ����дٽ����ã�����100mLNaOHʱ�����������Σ�ˮ��̶������a�㣻��b����Ҫ�����һЩNaOH�����c(Na��) >c(SO42-)>c(NH4+)>c(OH��)��c(H��)��
II����1��pH=9ʱpc��HCO3-��ߣ�Ũ�����2��c(H��)�����CO2��H2O
 H2CO3
H2CO3 H����HCO3-ƽ�������ƶ��ų�CO2��̼��Ũ�ȱ��ֲ��䣬pc=3����3����ΪM2CO3
H����HCO3-ƽ�������ƶ��ų�CO2��̼��Ũ�ȱ��ֲ��䣬pc=3����3����ΪM2CO3 2M��+CO32-����Ksp��c2(M��)��c(CO32-)�� (1��10��5) 2��c(CO32-)=1��10��12��c(CO32-)=1��10��2 mol��L��1����pc(CO32-)=2��
2M��+CO32-����Ksp��c2(M��)��c(CO32-)�� (1��10��5) 2��c(CO32-)=1��10��12��c(CO32-)=1��10��2 mol��L��1����pc(CO32-)=2��
��ϰ��ϵ�д�
�����Ŀ
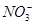 ��
��
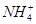 ��
��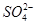 ��Cl��
��Cl��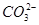 ��
��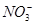 ��
�� ��
�� ��
�� �е����������ӡ�ijͬѧ�Ը���Һ��������ʵ�飺
�е����������ӡ�ijͬѧ�Ը���Һ��������ʵ�飺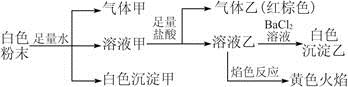
 ��Cl��
��Cl�� ��K����Cl����NO
��K����Cl����NO