��Ŀ����
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺��1����֪��N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol
����17g ��������������ȫ����һ�����������ˮ�������ų�������Ϊ
226.3kJ
226.3kJ
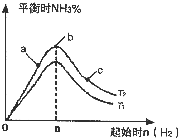
����2��ij����С���о����������������������£��ı���ʼ�����������ʵ�����N2��g��+3H2��g��?2NH3��g����Ӧ��Ӱ�죮
ʵ������ͼ��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����
��ͼ����T2��T1�Ĺ�ϵ�ǣ�T2
����
����
T1������ڡ������ڡ������ڡ�����ȷ���������ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������ߵ���
c
c
������ĸ������3��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��2N2O5��g��=4NO2��g��+O2��g����H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
| t/s | 0 | 500 | 1000 |
| c��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
0.00592mol?L-1?s-1
0.00592mol?L-1?s-1
����������1�����ݸ�˹��������Ӧ���ʱ䣻
��2���ٸ����¶ȶԻ�ѧƽ���ƶ���Ӱ��֪ʶ���ش�
�ڸ���a��b��c��������ʾ���������ش�
��3�����ݷ�Ӧ����v=
�����㣮
��2���ٸ����¶ȶԻ�ѧƽ���ƶ���Ӱ��֪ʶ���ش�
�ڸ���a��b��c��������ʾ���������ش�
��3�����ݷ�Ӧ����v=
| ��c |
| ��t |
����⣺��1����֪����N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol
��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol
���ø�˹���ɽ��١�2-�ڡ�2+�ۡ�3�ɵ�4NH3��g��+5O2��g��=4NO��g��+6H2O��g����
��H=��+180.5kJ?mol-1��2+92.4kJ?mol-1��2-483.6��3��=-905.2 kJ?mol-1��
17g��������������ȫ����һ�����������ˮ�������ų�������=
��905.2 kJ=226.3 kJ��
�ʴ�Ϊ��226.3 kJ��
��2���ٷ�ӦΪ���ȷ�Ӧ���¶�����ѧƽ���������ȷ�����У������¶Ȱ����ĺ������ͣ���T1��T2��
�ʴ�Ϊ�����ڣ�
��b�����ƽ��״̬��c���ּ�������������ƽ�������ƶ���������ת�������ʴ�Ϊ��c��
��3��500s��N2O5��Ũ�ȱ仯��Ϊ1.48mol?L-1����NO2��Ũ�ȱ仯��Ϊ2.96mol?L-1��
��v=
=
=0.00592mol?L-1?s-1��
�ʴ�Ϊ��0.00592 mol?L-1?s-1��
��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol
���ø�˹���ɽ��١�2-�ڡ�2+�ۡ�3�ɵ�4NH3��g��+5O2��g��=4NO��g��+6H2O��g����
��H=��+180.5kJ?mol-1��2+92.4kJ?mol-1��2-483.6��3��=-905.2 kJ?mol-1��
17g��������������ȫ����һ�����������ˮ�������ų�������=
| 1 |
| 4 |
�ʴ�Ϊ��226.3 kJ��
��2���ٷ�ӦΪ���ȷ�Ӧ���¶�����ѧƽ���������ȷ�����У������¶Ȱ����ĺ������ͣ���T1��T2��
�ʴ�Ϊ�����ڣ�
��b�����ƽ��״̬��c���ּ�������������ƽ�������ƶ���������ת�������ʴ�Ϊ��c��
��3��500s��N2O5��Ũ�ȱ仯��Ϊ1.48mol?L-1����NO2��Ũ�ȱ仯��Ϊ2.96mol?L-1��
��v=
| ��c |
| ��t |
| 2.96mol/L |
| 500s |
�ʴ�Ϊ��0.00592 mol?L-1?s-1��
���������⿼�鷴Ӧ�ȵļ����Լ���ѧƽ���ƶ����⣬ע����ո�˹���ɵ����ã�Ϊ��������ѵ㣬Ҳ���״��㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺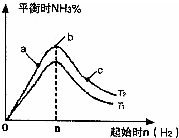 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺