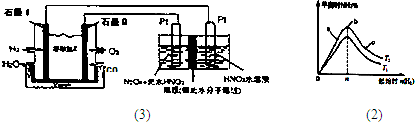
��Ŀ����
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺��1����֪��N2��g��+O2��g��=2NO��g����H=+180.5kJ/mol
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
д������������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
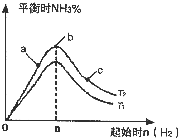
��2��ij����С���о����������������������£��ı���ʼ�����������ʵ����Է�ӦN2��g��+3H2��g��?2NH3��g����Ӱ�죮ʵ������ͼ��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����
��ͼ����T2��T1�Ĺ�ϵ�ǣ�T2
�ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������ߵ���
��3����һ���¶Ⱥʹ����£���3.2mol H2��1.2molN2�����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����2minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������0.8mol NH3�����㣺��д��������̣��������С�����һλ��
��2min����H2��ʾ�Ļ�ѧ��Ӧ���ʣ�
�ڸ������µ�ƽ�ⳣ����
��������1������֪�Ȼ�ѧ����ʽ���ݸ�˹���ɹ���Ŀ���Ȼ�ѧ����ʽ��
��2���ٸ����¶�����ѧƽ���������ȷ�����У�
�ڸ����������������ʵ�����ѧƽ����������Ӧ�����ƶ���
��3����������ʽ����������ʵ�Ũ�ȱ仯��ƽ��ʱ�����ʵ�Ũ�ȣ�
�ٸ���v=
������v��H2����
��ƽ�ⳣ��k=
����ƽ��Ũ�ȴ�����㣮
��2���ٸ����¶�����ѧƽ���������ȷ�����У�
�ڸ����������������ʵ�����ѧƽ����������Ӧ�����ƶ���
��3����������ʽ����������ʵ�Ũ�ȱ仯��ƽ��ʱ�����ʵ�Ũ�ȣ�
�ٸ���v=
| ��c |
| ��t |
��ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)?c3(H2) |
����⣺��1����֪����N2��g��+O2��g��=2NO��g����H=+180.5kJ/mol
��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
�ɸ�˹���ɿ�֪���١�2-�ڡ�2+�ۡ�3�ã�4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol��
��2���ٷ�ӦΪ���ȷ�Ӧ���¶�����ѧƽ���������ȷ�����У���T1��T2��Ӧ�ﵪ���������ӣ���T1��T2���ʴ�Ϊ�����ڣ�
��b�����ƽ��״̬��c���ּ�������������ƽ�������ƶ���������ת�������ʴ�Ϊ��c��
��3��2minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������0.8mol NH3��
N2��g��+3H2��g�� 2NH3��g����
2NH3��g����
��ʼ��mol����1.2 3.2 0
�仯��mol����0.4 1.2 0.8
ƽ�⣨mol����0.8 2 0.8
���Ԣ�v��H2��=
=
=
=0.3mol/��L?min����
�ʴ�Ϊ��0.3mol/��L?min����
��ƽ�ⳣ��k=
=
=0.4��L2/mol2����
�ʴ�Ϊ��0.4��L2/mol2����
��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
�ɸ�˹���ɿ�֪���١�2-�ڡ�2+�ۡ�3�ã�4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol��
��2���ٷ�ӦΪ���ȷ�Ӧ���¶�����ѧƽ���������ȷ�����У���T1��T2��Ӧ�ﵪ���������ӣ���T1��T2���ʴ�Ϊ�����ڣ�
��b�����ƽ��״̬��c���ּ�������������ƽ�������ƶ���������ת�������ʴ�Ϊ��c��
��3��2minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������0.8mol NH3��
N2��g��+3H2��g��
 2NH3��g����
2NH3��g������ʼ��mol����1.2 3.2 0
�仯��mol����0.4 1.2 0.8
ƽ�⣨mol����0.8 2 0.8
���Ԣ�v��H2��=
| ��c(H2) |
| ��t |
| ||
| ��t |
| ||
| 2min |
�ʴ�Ϊ��0.3mol/��L?min����
��ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)?c3(H2) |
(
| ||||
(
|
�ʴ�Ϊ��0.4��L2/mol2����
���������⿼�����Ȼ�ѧ����ʽ��д��Ӱ��ƽ���ƶ������ء�ƽ��ͼ��ѧƽ�����ȣ��Ѷ��еȣ�ע����������ʽ���ⷨ���˹���ɣ�

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺ ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺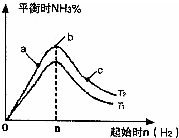 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺