��Ŀ����
SNCR��SCR��һ�����͵�������������(��ȥ�����е�NOx�������������£�

��1����Ӧ2NO��2CO 2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
��2��SNCR��SCR�����з�������Ҫ��Ӧ�У�
��4NO(g)��4NH3(g)��O2(g) 4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1��
4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1��
��6NO(g)��4NH3(g) 5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1��
5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1��
��6NO2(g)��8NH3(g) 7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1��
7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1��
��ӦN2(g)��O2(g) 2NO(g)�Ħ�H�� kJ?mol��1��
2NO(g)�Ħ�H�� kJ?mol��1��
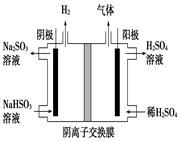
��3��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ��

�õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦΪ��
��4�������øõ�ش�����ҵ��ˮ�к��е�Cr2O72����������������Fe��������⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�����������ҺpH���ߣ�����Cr��OH��3��������ȥCr2O72-��
��д����������Cr2O72������ԭΪCr3+�����ӷ���ʽ�� ��
�ڸõ�ع���ʱÿ����100L Cr2O72-Ũ��Ϊ0.002mol/L��ˮ�����ı�״�������� L��

��1����Ӧ2NO��2CO
 2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0�����������������2��SNCR��SCR�����з�������Ҫ��Ӧ�У�
��4NO(g)��4NH3(g)��O2(g)
 4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1��
4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1����6NO(g)��4NH3(g)
 5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1��
5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1����6NO2(g)��8NH3(g)
 7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1��
7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1����ӦN2(g)��O2(g)
 2NO(g)�Ħ�H�� kJ?mol��1��
2NO(g)�Ħ�H�� kJ?mol��1����3��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ��

�õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦΪ��
��4�������øõ�ش�����ҵ��ˮ�к��е�Cr2O72����������������Fe��������⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�����������ҺpH���ߣ�����Cr��OH��3��������ȥCr2O72-��
��д����������Cr2O72������ԭΪCr3+�����ӷ���ʽ�� ��
�ڸõ�ع���ʱÿ����100L Cr2O72-Ũ��Ϊ0.002mol/L��ˮ�����ı�״�������� L��
��1��< (2��)��2��+179.8 (2��) ��3��NO2��NO3����e����N2O5(2��)
��4����Cr2O72��+ 6Fe2++ 14H+= 2Cr3++ 6Fe3++7 H2O (2��)��13.44 (2��)
��4����Cr2O72��+ 6Fe2++ 14H+= 2Cr3++ 6Fe3++7 H2O (2��)��13.44 (2��)
�����������1��Ҫ�뷴Ӧ2NO��2CO
 2CO2��N2�ܹ��Է����У����Ц�H��T��S��0���÷�ӦΪ�������ʵ�����С���ؼ���Ӧ����S��0����÷�Ӧ�Ħ�H��0����2�����ݸ�˹���ɣ��١��ڵ�
2CO2��N2�ܹ��Է����У����Ц�H��T��S��0���÷�ӦΪ�������ʵ�����С���ؼ���Ӧ����S��0����÷�Ӧ�Ħ�H��0����2�����ݸ�˹���ɣ��١��ڵ�N2(g)��O2(g)
 2NO(g)�Ħ�H��+179.8kJ?mol��1����3�������ȼ�ϵ��װ��ͼ֪��NO2�ڸ�������������Ӧ������N2O5,�缫��ӦʽΪ��NO2��NO3����e����N2O5����4������Fe��������⺬Cr2O72-�����Է�ˮ�������缫��ӦʽΪ��Fe - 2e-=Fe2+����������Cr2O72������ԭΪCr3+�����ӷ���ʽΪ
2NO(g)�Ħ�H��+179.8kJ?mol��1����3�������ȼ�ϵ��װ��ͼ֪��NO2�ڸ�������������Ӧ������N2O5,�缫��ӦʽΪ��NO2��NO3����e����N2O5����4������Fe��������⺬Cr2O72-�����Է�ˮ�������缫��ӦʽΪ��Fe - 2e-=Fe2+����������Cr2O72������ԭΪCr3+�����ӷ���ʽΪCr2O72��+ 6Fe2++ 14H+= 2Cr3++ 6Fe3++7 H2O���ڸ��������Ӧ�͵����غ��Cr2O72���������Ĺ�ϵʽ��Cr2O72������3O2��100L Cr2O72-Ũ��Ϊ0.002mol/L��ˮ��Cr2O72-�����ʵ���Ϊ0.2mol����������Ϊ0.6mol����״���µ����Ϊ13.44L��

��ϰ��ϵ�д�
�����Ŀ
 N2(g)��CO2(g)��2H2O(g)����H����867 kJ��mol��1���÷�Ӧ���������������������Ⱦ����130 ���180 ��ʱ���ֱ�0.50 mol CH4��a mol NO2����1 L���ܱ������з�����Ӧ������й��������±���
N2(g)��CO2(g)��2H2O(g)����H����867 kJ��mol��1���÷�Ӧ���������������������Ⱦ����130 ���180 ��ʱ���ֱ�0.50 mol CH4��a mol NO2����1 L���ܱ������з�����Ӧ������й��������±���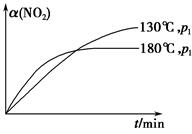

 H= -Q1 kJ/mol��
H= -Q1 kJ/mol��  CO(g)+H2(g)����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
CO(g)+H2(g)����÷�Ӧ��ƽ�ⳣ������ʽΪ ��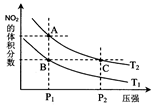
 2NO2(g);��H=" +57.0" kJ��mol-1��NO2��N2O4��Ũ����ͼ����ʾ��NO2��N2O4��������������Ũ�ȵĹ�ϵ����ͼ��ʾ��
2NO2(g);��H=" +57.0" kJ��mol-1��NO2��N2O4��Ũ����ͼ����ʾ��NO2��N2O4��������������Ũ�ȵĹ�ϵ����ͼ��ʾ��
 2SO3��ƽ�ⳣ�����±���
2SO3��ƽ�ⳣ�����±���

