��Ŀ����
��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ����ͼ��ʾ��

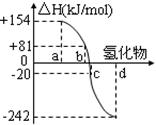
��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol������������)��

(1)д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ_____________________��
(2)д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ_________________________��
(3)����֪SO2���������ļ���Ϊd kJ��mol-1��O2���������ļ���Ϊe kJ��mol-1����S8������������ļ���Ϊ___________��

��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol������������)��

(1)д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ_____________________��
(2)д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ_________________________��
(3)����֪SO2���������ļ���Ϊd kJ��mol-1��O2���������ļ���Ϊe kJ��mol-1����S8������������ļ���Ϊ___________��
(1)S8(s)+8O2(g)==8SO2(g) ��H="-8a" kJ�Mmol
(2)2SO3(g) 2SO2(g)+O2(g) ��H=+2bkJ�Mmol
2SO2(g)+O2(g) ��H=+2bkJ�Mmol
(3)(2d-a-e) kJ�Mmol
(2)2SO3(g)
 2SO2(g)+O2(g) ��H=+2bkJ�Mmol
2SO2(g)+O2(g) ��H=+2bkJ�Mmol(3)(2d-a-e) kJ�Mmol
�����������ͼ�ɿ�������1mol�Ķ�������ų�����ΪaKJ�Mmol������S8ȼ�յ��Ȼ�ѧ����ʽΪ�� S8��s��+8O2(g)=8SO2(g ) ; ��H=-8aKJ�Mmol (2) SO2��O2�������ߣ�SO3�����͡�SO3�ֽ�����SO2��O2�������ֽ�2���Ȼ�ѧ����ʽΪ��2SO3(g)
 2SO2(g)+O2(g) ��H="+2b" kJ�Mmol.���ݷ�Ӧ�Ⱦ��Ƕ��Ѿɼ����յ��������γ��»�ѧ�����ͷŵ������IJ�ֵ������S��S��ΪX����8X+8e-16d="-8a" ,���X=��2d-a-e��kJ�Mmol.
2SO2(g)+O2(g) ��H="+2b" kJ�Mmol.���ݷ�Ӧ�Ⱦ��Ƕ��Ѿɼ����յ��������γ��»�ѧ�����ͷŵ������IJ�ֵ������S��S��ΪX����8X+8e-16d="-8a" ,���X=��2d-a-e��kJ�Mmol.
��ϰ��ϵ�д�
�����Ŀ

 2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
 O2(g)=H2O(l) ��H3����285.8 kJ/mol
O2(g)=H2O(l) ��H3����285.8 kJ/mol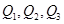 ���߹�ϵ��ȷ����
���߹�ϵ��ȷ����
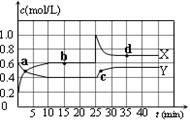
 N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��
N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��