��Ŀ����
����Ŀ����.�������(K2FeO4)���м�ǿ�������ԣ���һ��������ˮ��������
��1�������FeO42-��ˮ��Ӧ�����ӷ���ʽ��4FeO42-��10H2O![]() 4Fe(OH)3��8OH����__��K2FeO4�ڴ���ˮ�Ĺ����������������___��____��
4Fe(OH)3��8OH����__��K2FeO4�ڴ���ˮ�Ĺ����������������___��____��
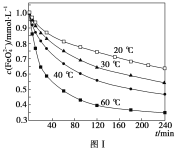
��2��������K2FeO4���Ƴ�c(FeO42-)��1.0mmol��L��1���������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42-)�ı仯�������ͼ�ڣ�1�����еķ�ӦΪFeO42-�仯������Ӧ�����¶ȶԸ÷�Ӧ�ķ�Ӧ���ʺ�ƽ���ƶ���Ӱ����___��������Ӧ����H___0��
��.�±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp(25��)��
����� | ƽ�ⷽ��ʽ | ƽ�ⳣ��K | Ksp |
CH3COOH | CH3COOH | 1.76��10��5 | |
H2CO3 | H2CO3 HCO3- | K1=4.31��10��4 K2=5.61��10��11 | |
C6H5OH | C6H5OH | 1.1��10��10 | |
H3PO4 | H3PO4 H2PO4- | K1=7.52��10��3 K2=6.23��10��8 K3=2.20��10��13 | |
NH3��H2O | NH3��H2O | 1.76��10��5 | |
BaSO4 | BaSO4 | 1.07��10��10 | |
BaCO3 | BaCO3 | 2.58��10��9 |
�ش��������⣺
��1�����ϱ�����������CH3COOH����HCO3-����C6H5OH����H2PO4-���ɿ����ᣬ������������ǿ������˳��Ϊ___(����)��
��2��д��C6H5OH��Na3PO4��Ӧ�����ӷ���ʽ___��
��3��25��ʱ�����������Ũ�ȵĴ���Ͱ�ˮ��ϣ����Һ�У�c(CH3COO��)__c(NH4+)(����>��������������<��)��
��4��25��ʱ����10mL0.01mol��L��1������Һ�еμ�VmL0.01mol��L��1��ˮ�����Һ������Ũ�ȹ�ϵ��ȷ����__��
A�������ҺpH>7����V��10
B�������ҺpH<7����c(NH4+)>c(C6H5O��)>c(H��)>c(OH��)
C��V��10ʱ�����Һ��ˮ�ĵ���̶�С��10mL0.01mol��L��1������Һ��ˮ�ĵ���̶�
D��V��5ʱ��2c(NH3��H2O)��2c(NH4
��5��ˮ�ⷴӦ�ǵ��͵Ŀ��淴Ӧ��ˮ�ⷴӦ�Ļ�ѧƽ�ⳣ����Ϊˮ�ⳣ��(��Kb��ʾ)����Ȼ�ѧƽ�ⳣ���Ķ��壬��д��Na2CO3��һ��ˮ�ⷴӦ��ˮ�ⳣ���ı���ʽ��___��
���𰸡�3O2 ɱ������ ����(��ˮ) �¶����ߣ���Ӧ���ʼӿ죬ƽ��������Ӧ�����ƶ� > �٢ܢۢ� C6H5OH��PO43-=C6H5O����HPO42- = D Kb=![]()
��������
��.��1��FeO42-��ˮ��Ӧ�����ӷ���ʽ��4FeO42-��10H2O![]() 4Fe(OH)3��8OH����__����Ӧ�У�Fe��+6�۽���Ϊ+3�ۣ����̬���ߵ�Ԫ��ֻ��ΪH2O�е�O���Ӷ��ó���������ΪO2��K2FeO4�ڴ���ˮ�Ĺ����У�Fe��̬���ͣ���������������Fe(OH)3���壬����ˮ����
4Fe(OH)3��8OH����__����Ӧ�У�Fe��+6�۽���Ϊ+3�ۣ����̬���ߵ�Ԫ��ֻ��ΪH2O�е�O���Ӷ��ó���������ΪO2��K2FeO4�ڴ���ˮ�Ĺ����У�Fe��̬���ͣ���������������Fe(OH)3���壬����ˮ����
��2����ͼ�п��Կ����������¶ȣ�c(FeO42-)��С����ƽ�������ƶ���
��. ��1�����ϱ�����������CH3COOH����HCO3-����C6H5OH����H2PO4-���ɿ����ᣬ���еĵ��볣���ֱ�Ϊ��1.76��10��5��5.61��10��11��1.1��10��10��6.23��10��8����ȷ������������ǿ������˳��
��2��C6H5OH��Na3PO4��Ӧ������C6H5O-��HPO42-��
��3��25��ʱ�����������Ũ�ȵĴ���Ͱ�ˮ��ϣ����ڶ��ߵĵ��볣����ͬ�����Ի��Һ�У�CH3COO����NH4+��ˮ��̶�Ҳ��ͬ���ɴ˿ɵó�c(CH3COO��)��c(NH4+)�Ĵ�С��ϵ��
��4��A���ɶ��ߵĵ��볣��������������ҺpH>7��Ҳ�п��ܳ���V<10��
B�������ҺpH<7����Ӧ����C6H5ONH4��ʣ���C6H5OH�ַ������룬�Ӷ�ʹ��Һ�����ԣ�
C��V��10ʱ�����߸պ���ȫ��Ӧ�����Һ�з���C6H5O����NH4+��ˮ�ⷴӦ��ˮ�ĵ���̶ȴ��ڱ�����Һ��ˮ�ĵ���̶ȣ�
D��V��5ʱ��n(C6H5OH)=2n(NH3��H2O)�������غ�ԭ���֪��2c(NH3��H2O)��2c(NH4+)��c(C6H5O��)��c(C6H5OH)��
��5��Na2CO3��һ��ˮ�ⷴӦΪCO32-��H2O![]() HCO3-��OH-���ݴ�д��ˮ�ⳣ���ı���ʽ��
HCO3-��OH-���ݴ�д��ˮ�ⳣ���ı���ʽ��
��.��1��FeO42-��ˮ��Ӧ�����ӷ���ʽ��4FeO42-��10H2O![]() 4Fe(OH)3��8OH����3O2����Ӧ�У�Fe��+6�۽���Ϊ+3�ۣ����̬���ߵ�Ԫ��ֻ��ΪH2O�е�O���Ӷ��ó���������ΪO2��K2FeO4�ڴ���ˮ�Ĺ����У�Fe��̬���ͣ�������������ɱ���������ã�����Fe(OH)3���壬����ˮ������Ϊ��3O2��ɱ������������(��ˮ)��
4Fe(OH)3��8OH����3O2����Ӧ�У�Fe��+6�۽���Ϊ+3�ۣ����̬���ߵ�Ԫ��ֻ��ΪH2O�е�O���Ӷ��ó���������ΪO2��K2FeO4�ڴ���ˮ�Ĺ����У�Fe��̬���ͣ�������������ɱ���������ã�����Fe(OH)3���壬����ˮ������Ϊ��3O2��ɱ������������(��ˮ)��
��2����ͼ�п��Կ����������¶ȣ�c(FeO42-)��С����ƽ�������ƶ����Ӷ��ó��¶ȶԸ÷�Ӧ�ķ�Ӧ���ʺ�ƽ���ƶ���Ӱ�����¶����ߣ���Ӧ���ʼӿ죬ƽ��������Ӧ�����ƶ���������Ӧ����H>0����Ϊ���¶����ߣ���Ӧ���ʼӿ죬ƽ��������Ӧ�����ƶ���>��
��. ��1�����ϱ�����������CH3COOH����HCO3-����C6H5OH����H2PO4-���ɿ����ᣬ���еĵ��볣���ֱ�Ϊ��1.76��10��5��5.61��10��11��1.1��10��10��6.23��10��8����ȷ������������ǿ������˳��Ϊ�٢ܢۢڡ���Ϊ���٢ܢۢڣ�
��2��C6H5OH��Na3PO4��Ӧ������C6H5O-��HPO42-����Ӧ�����ӷ���ʽΪC6H5OH��PO43-=C6H5O����HPO42-����Ϊ��C6H5OH��PO43-=C6H5O����HPO42-��
��3��25��ʱ�����������Ũ�ȵĴ���Ͱ�ˮ��ϣ����ڶ��ߵĵ��볣����ͬ�����Ի��Һ�У�CH3COO����NH4+��ˮ��̶�Ҳ��ͬ���ɴ˿ɵó����Һ�У�c(CH3COO��)=c(NH4+)����Ϊ��=��
��4��A���ɶ��ߵĵ��볣��������������ҺpH>7��Ҳ�п��ܳ���V<10��A����ȷ��
B�������ҺpH<7����Ӧ����C6H5ONH4��ʣ���C6H5OH�ַ������룬�Ӷ�ʹ��Һ�����ԣ��ɴ˿ɵó�c(C6H5O��)>c(NH4+)> c(H��)>c(OH��)��B����ȷ��
C��V��10ʱ�����߸պ���ȫ��Ӧ�����Һ�з���C6H5O����NH4+��ˮ�ⷴӦ��ˮ�ĵ���̶ȴ���10mL0.01mol��L��1������Һ��ˮ�ĵ���̶ȣ�C����
D��V��5ʱ��n(C6H5OH)=2n(NH3��H2O)�������غ�ԭ��2c(NH3��H2O)��2c(NH4+)��c(C6H5O��)��c(C6H5OH)��D��ȷ����Ϊ��D��
��5��Na2CO3��һ��ˮ�ⷴӦΪCO32-��H2O![]() HCO3-��OH-��ˮ�ⳣ���ı���ʽΪ
HCO3-��OH-��ˮ�ⳣ���ı���ʽΪ![]() ����Ϊ��
������![]() ��
��

����Ŀ������ʵ������ܴﵽʵ��Ŀ����( )
ʵ��Ŀ�� | ʵ����� | |
A | ��ȥ | �������� |
B | ������Һ���Ƿ��� | ȡ������Һ���Թ��У��ȼ��� |
C | �Ƚ�HCl�� | ��pH��ֽ�ⶨŨ�Ⱦ�Ϊ |
D | ��֤ | �������ữ�� |
A.AB.BC.CD.D