��Ŀ����
����Ŀ��(1)�谢���ӵ�����ΪNA����״���£�ijO2��N2�Ļ������m g����b�����ӣ���ng�û����������ͬ״������ռ�������___L��
(2)��xR2++yH++O2�TmR3++nH2O�����ӷ���ʽ�У��Ի�ѧ������m��R2+��R3+�ж���ȷ����___��
A.m=y��R3+���������� B.m=2y��R2+������
C.m=2��R3+�������� D.m=4��R2+�ǻ�ԭ��
(3)��˫���ŷ�������з�Ӧ�ĵ���ת�Ʒ������Ŀ��___
2KMnO4+16HCl(Ũ)=2KCl+2MnCl2+5Cl2��+8H2O
���𰸡�![]() AD
AD 
��������
(1)�����ng�û�����庬�еķ��������ٸ���n=![]() �����ng�û���������ʵ�����������V=n��Vm�����n g�û����������ͬ״������ռ�������
�����ng�û���������ʵ�����������V=n��Vm�����n g�û����������ͬ״������ռ�������
(2)���ݷ�Ӧǰ�����Ԫ�ص�ԭ�Ӹ�����ȣ��ɵ�m��n��x��y����ֵ����ϵ���غ�ȷ��2x+y=3m������x=y=m���ٸ���������ԭ��Ӧ�Ĺ����ж����ʵ����ã��Դ������
(3)��Ӧ2KMnO4+16HCl(Ũ)=2KCl+2MnCl2+5Cl2��+8H2O�У���Ԫ�صĻ��ϼ���+7�۱��+2�ۣ������в�������-1�۱��0�ۣ��ɴ���˫���ŷ��������ת�Ƶķ������Ŀ��
(1)��״���»�������к������䣬����������������������ȣ���ng�û�����庬�еķ�����Ϊx����������������ı���ʽΪ��mg��b=ng��x�����x=![]() ����ng�û�����庬�е����ʵ���Ϊ��n=
����ng�û�����庬�е����ʵ���Ϊ��n=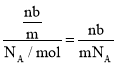 mol���������ڱ�״���µ����Ϊ��V=n��Vm=
mol���������ڱ�״���µ����Ϊ��V=n��Vm=![]() L���ʴ�Ϊ��
L���ʴ�Ϊ��![]() ��
��
(2)������ԭ���غ㣬����n=2��������ԭ���غ㣬����y=4������Rԭ���غ���x=m�����ݵ���غ���2x+y=3m������x=y=m���ڷ�Ӧ�У�R�Ļ��ϼ����ߣ�R2+�ǻ�ԭ��������R3+�����������Ԫ�ػ��ϼ۽��ͣ�����H2O�ǻ�ԭ���
A.m=y=4��R3+���������A��ȷ��
B.m=y��R2+�ǻ�ԭ������������B����
C.m=4��R3+���������C����
D.m=4��R2+�ǻ�ԭ����D��ȷ��
AD��ȷ���ʴ�Ϊ��AD��
(3)��Ӧ2KMnO4+16HCl(Ũ)=2KCl+2MnCl2+5Cl2��+8H2O�У���Ԫ�صĻ��ϼ��ɷ�ӦǰKMnO4�е�+7�۱�ɷ�Ӧ��MnCl2�е�+2�ۣ����ϼ۽��ͣ������в������ɷ�ӦǰHCl�е�-1�۱�ɷ�Ӧ��Cl2�е�0�ۣ����ϼ����ߣ�����Ԫ�ػ��ϼ������������ڷ�Ӧ������ʧȥ��õ��ĵ�����������˫���ŷ��������ת�Ƶķ������ĿΪ��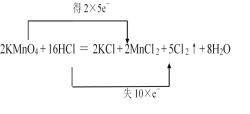 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 ��У����ϵ�д�
��У����ϵ�д�