��Ŀ����
����Ŀ������������(H2N2O2)��һ�ֶ�Ԫ�ᣬ��������N2O���塣
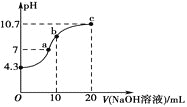
��1�������£���0.01mol��L-1��NaOH��Һ�ζ�10mL0.01mol��L-1H2N2O2��Һ�������ҺpH��NaOH��Һ����Ĺ�ϵ��ͼ��ʾ��
��д��H2N2O2��ˮ��Һ�еĵ��뷽��ʽ��__��
��c��ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ__��
��b��ʱ��Һ��c(H2N2O2)__c(N2O22-)��(������������������=������ͬ)
��a��ʱ��Һ��c(Na+)__c(HN2O)+c(N2O22-)��
��2����������Һ����������������Һ��ϣ����Եõ���ɫ����������������������÷�ɢϵ�еμ���������Һ������ɫ�����ͻ�ɫ��������ʱ����ɢϵ��![]() =__��[��֪Ksp(Ag2N2O2)=4.2��10-9��Ksp(Ag2SO4)=1.4��10-5]
=__��[��֪Ksp(Ag2N2O2)=4.2��10-9��Ksp(Ag2SO4)=1.4��10-5]
��3����ʱ���ǽ�NaHCO3��Һ�е�ƽ���ʾΪ��2HCO3-![]() H2CO3+CO32-��Ϊ��֤����ƽ����ڣ�����ΪӦ��NaHCO3��Һ�м������������Լ�����___������ţ�
H2CO3+CO32-��Ϊ��֤����ƽ����ڣ�����ΪӦ��NaHCO3��Һ�м������������Լ�����___������ţ�
A.�ʵ�Ũ�ȵ�����
B.�ʵ�Ũ��Ba(OH��2��Һ
C.�ʵ�Ũ��BaCl2��Һ
���𰸡�H2N2O2![]() HN2O2-+H+ c(Na+��c(N2O22-)��c(OH-)��c(HN2O2-)��c(H+) �� �� 3��10-4 C
HN2O2-+H+ c(Na+��c(N2O22-)��c(OH-)��c(HN2O2-)��c(H+) �� �� 3��10-4 C
��������
��������20mLNaOH��Һ��ǡ����ȫ��Ӧ������Na2N2O2��������Һ��pH=10.7���Լ��ԣ���Һ�д���N2O22-��ˮ�⣬˵��H2N2O2Ϊ�����c����Һ������ΪNa2N2O2����Һ�д���N2O22��ˮ�⣬��˵���Һ������Ũ�ȹ�ϵ����b����Һ������ΪNaHN2O2����Һ�д���HN2O2���ĵ����ˮ�⣬������Һ�Լ��ԣ�˵��HN2O2����ˮ����ڵ��룻��a����Һ�����ԣ����ݵ���غ�ͳ����Եó��𰸡�
�Ƹ���ƽ�ⳣ�����Լ��㡣
��A. �����ʵ�Ũ�ȵ����ᣬ��֤����Һ�д���CO32��B. ����Ba(OH)2��Һ��OH-����HCO3����Ӧ����CO32����֤����Һ�д���CO32��C. ����BaCl2��Һ��������ɫ������˵����Һ�д���CO32��
��������20mLNaOH��Һ��ǡ����ȫ��Ӧ������Na2N2O2��������Һ��pH=10.7���Լ��ԣ���Һ�д���N2O22-��ˮ�⣬˵��H2N2O2Ϊ���ᣬ����ˮ�в��ֵ��룬����뷽��ʽΪ��H2N2O2HN2O2��+H+���ʴ�Ϊ��H2N2O2HN2O2��+H+��
��c����Һ������ΪNa2N2O2����Һ�д���N2O22��ˮ�⣬�����Һ������Ũ�ȹ�ϵΪ��c(Na+)��c(N2O22)��c(OH��)��c(HN2O2��)��c(H+)���ʴ�Ϊ��c(Na+)��c(N2O22)��c(OH��)��c(HN2O2��)��c(H+)��
��b����Һ������ΪNaHN2O2����Һ�д���HN2O2���ĵ����ˮ�⣬������Һ�Լ��ԣ�˵��HN2O2����ˮ����ڵ��룬�����Һ��c(H2N2O2)>c(N2O22)���ʴ�Ϊ������
��a����Һ�����ԣ���Һ��c(H+)=c(OH��)����Һ�д��ڵ���غ�c(Na+)+c(H+)=c(OH��)+ c(HN2O2��)+2c(N2O22)������Һ��c(Na+)=c(HN2O2��)+2c(N2O22)�������Һ��c(Na+)> c(HN2O2��) + c(N2O22-)���ʴ�Ϊ������
��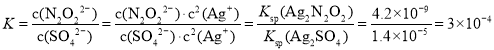 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��A. �����ʵ�Ũ�ȵ����ᣬ��֤����Һ�д���CO32����A���������⣻B. ����Ba(OH)2��Һ��OH-����HCO3����Ӧ����CO32����֤����Һ�д���CO32����B���������⣻C. ����BaCl2��Һ��������ɫ������˵����Һ�д���CO32����C�������⣻������������ΪC��

����Ŀ���ּס�������ѧС�鰲װ������ͼ��ʾ����ͬװ�ã�����̽��Ӱ��H2O2�ֽ����ʵ����ء�

��1������a������___��
��2��MnO2����H2O2�ֽ�Ļ�ѧ����ʽ��___��
��3����С��������ʵ����Ʒ����������������ɱ�����δ��֡�
ʵ���� | ʵ��Ŀ�� | T/K | ���� | Ũ�� |
����ʵ��� | ��ʵ����� | 298 | 3��FeCl3��Һ | 10mL2%H2O2 |
����ʵ��� | ��__ | 298 | ��__ | 10mL5%H2O2 |
��4���ס�����С��ó���ͼ���ݡ�
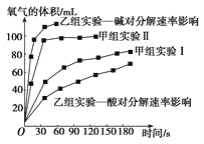
���ɼ���ʵ�����ݿɵó����ֽ���__��
���������о����ᡢ���H2O2�ֽ�Ӱ�����ص�������ͬ�����£�Na2O2��K2O2����ˮ�ų��������ʽϿ����__���������������BaO2������H2SO4��Һ��Ӧ��H2O2���仯ѧ��Ӧ����ʽΪ_��֧����һ������������_��
����Ŀ��X��Y��Z ����ѧ��ѧ�г������������ʣ��±���������֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת����ϵ����
X | Y | Z | ��ͷ���������ֵķ�Ӧ���� | ||
A�� | NO | NO2 | HNO3 | �ٳ��������� |
|
B�� | Cl2 | NaClO | HClO | ��ͨ��CO2 | |
C�� | Na2O2 | NaOH | NaCl | �ۼ���H2O2 | |
D�� | Al2O3 | NaAlO2 | Al(OH)3 | �ܼ�NaOH��Һ |
A. A B. B C. C D. D