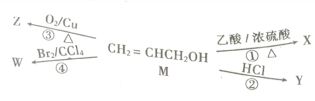��Ŀ����
����Ŀ�������������������ʻ�����ijѧϰС��ͨ��ʵ��̽��һ�ֵ����ʵ�Ԫ����ɡ�
I.ȷ���õ������е�ijЩ���Ԫ��
��1��Ϊȷ���õ������к���Ԫ��������Ʒ���л���ת������Σ���֤����δ��ڵ�ʵ�鷽����_________________��
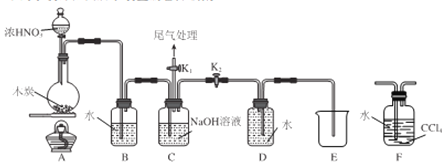
��2��Ϊȷ���õ������к�̼���⡢������Ԫ�أ���������ͼװ�ý����о���ͨ������ʹ��Ʒ��װ��A�г��ȼ��,��ʹ��������λ���ͨ������װ�á�

��װ��B�е��Լ���_________��
��װ��D��������____________________��
����װ��B��C��E��F���γ�����������:________________,Ʒ����ɫ��______________�����ְ�ɫ���ǣ���֤��ȼ�ղ����к���H2O��SO2��CO2��
����:�õ������к�̼���⡢����Ԫ�ء�
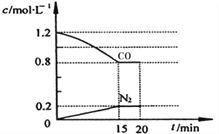
II.Ϊ�ⶨ�õ���������Ԫ�ص�����������С��ȡ��������Ʒ���ȼ�գ�����������ˮ���ն���������ȡ����Һ���Ե���Ϊָʾ�����������������Һ�ζ������ĵ⡣��֪:2S2032-+I2=S4062-+2I-
��3��д�������������ˮ��Ӧ�Ļ�ѧ����ʽ:___________________________��
��4���ζ��յ������Ϊ:______________________��
��5��ȡ��������Ʒmg���вⶨ������C1mol/L�ĵ�ˮV1mL�������գ��ζ������ĵ�ʱ����C2mol/L�����������ҺV2mL���õ������е���Ԫ�ص���������Ϊ______________��
��6����ȼ��ʱ����������������Һ�У����ܻᵼ�¸õ������е���Ԫ�ص����������ⶨֵ___(�ƫ��������ƫС��������Ӱ����)��
���𰸡� ע������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ������ ��ˮ����ͭ ��ȥSO2�������CO2�ļ�����ɸ��� ��ĩ�ɰ�ɫ����ɫ ����ɫ�������Ժ�ɫ�� I2��SO2��2H2O = H2SO4��2HI ���������һ��Na2S2O3��Һ����Һ����ɫ��ȥ���Ұ�����ڲ��ָ�ԭɫ ![]() ƫС
ƫС
�����������������I.��1������笠����ӵļ��鷽���ش�����2���������к�̼���⡢������Ԫ�أ�ͨ������ʹ��Ʒ��װ��A�г��ȼ�������ɶ�����̼��ˮ������������Bװ����ʢ����ˮ����ͭ����ˮ��Cװ���е�Ʒ��������������Dװ���еĸ��������Һ������������Eװ���е�Ʒ�������������Ƿ������F�еij���ʯ��ˮ���������̼��II. ��3�������������ˮ��Ӧ�������������������4���ﵽ�ζ��յ�ʱ�ⵥ����ȫ����ԭΪI-����5�����ݵ����غ�����Ԫ�ص�������������6������������������Һ�У����ֶ��������������������������������Һ�����ƫ����
������I.��1��ע������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ����������֤�������������2���������к�̼���⡢������Ԫ�أ�ͨ������ʹ��Ʒ��װ��A�г��ȼ�������ɶ�����̼��ˮ����������Bװ����ʢ����ˮ����ͭ����ˮ��Cװ���е�Ʒ��������������Dװ���еĸ��������Һ������������Eװ���е�Ʒ�������������Ƿ������F�еij���ʯ��ˮ���������̼��������װ��B�е��Լ�����ˮ����ͭ����װ��D�������dz�ȥSO2�������CO2�ļ�����ɸ���������װ��B��C��E��F���γ�����������: ��ĩ�ɰ�ɫ����ɫ��C��Ʒ����ɫ��E��Ʒ�첻��ɫ�������Ժ�ɫ����F�г��ְ�ɫ���ǣ���֤��ȼ�ղ����к���H2O��SO2��CO2��II. ��3�������������ˮ��Ӧ�������������������Ӧ����ʽ��I2��SO2��2H2O = H2SO4��2HI����4���ﵽ�ζ��յ�ʱ�ⵥ����ȫ����ԭΪI-�������ǵ��������һ��Na2S2O3��Һ����Һ����ɫ��ȥ���Ұ�����ڲ��ָ�ԭɫ����5����mg��Ʒ����Ԫ�ص�������xg�����ݵ����غ�![]() ��
�� ![]() ���õ������е���Ԫ�ص���������Ϊ
���õ������е���Ԫ�ص���������Ϊ![]() =
=![]() ����6������������������Һ�У����ֶ��������������������������������Һ�����ƫ�������Ե������е���Ԫ�ص����������ⶨֵƫС��
����6������������������Һ�У����ֶ��������������������������������Һ�����ƫ�������Ե������е���Ԫ�ص����������ⶨֵƫС��

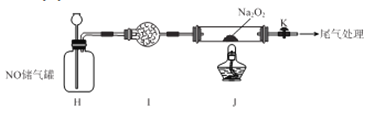
����Ŀ��ij�о���ѧϰС��ͨ�����з�Ӧԭ���Ʊ�SO2����������̽������Ӧԭ��Ϊ��Na2SO3(��)+H2SO4(Ũ)�� Na2SO4 + SO2��+ H2O

��1����������ԭ���Ʊ����ռ�����SO2��ʵ��װ������˳��Ϊ________________��(���ͷ���)
��2��D��ʢװ���Լ�Ϊ_________________��
��3����ͬѧ��ע������ȡ������SO2�����װ��G����SO2������ʵ�飬��X��Na2S��Һ����Ŀ���Ǽ���SO2��_____________���ɹ۲쵽������_________ ��
��4��ʵ��1����ͬѧ����ҺX��ΪŨ�Ⱦ�Ϊ0.1mol/L Fe(NO3)3��BaCl2�Ļ����Һ���Ѿ���ȥ�ܽ�������ͨ������SO2��۲쵽�ձ�������ɫ��������ͬѧ��Ϊ��ɫ����ΪBaSO4��Ϊ̽����ɫ�����ij�����������������ʵ����֤������֪��0.1mol/L Fe(NO3) 3��pH=2��
ʵ�� | ���� | ���� | ���ۺͽ��� |
2 | ��SO2ͨ��0.1mol/L ____��BaCl2���Һ | ������ɫ���� | Fe3+������H2SO3 |
3 | ��SO2ͨ��_______ �� BaCl2���Һ | ������ɫ���� | ��������NO3-�ܽ�H2SO3����ΪSO42- |