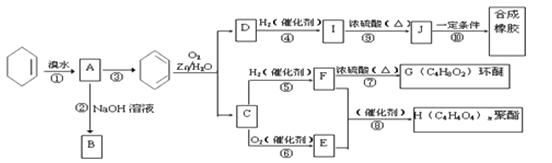
��Ŀ����
����Ŀ�����¶�T1��T2ʱ���ֱ�0.50 mol CH4��1.20 mol NO2�������Ϊ1 L���ܱ����������������·�Ӧ��CH4(g)��2NO2(g)![]() N2(g)��CO2(g)��2H2O(g)�����n(CH4)��ʱ��仯�������±���
N2(g)��CO2(g)��2H2O(g)�����n(CH4)��ʱ��仯�������±���
ʱ��/min | 0 | 10 | 20 | 40 | 50 | |
T1 | n(CH4)/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
T2 | n(CH4)/mol | 0.50 | 0.30 | 0.18 | ���� | 0.15 |
����˵����ȷ����(����)
A. T1ʱ0��10 min NO2��ƽ����Ӧ����Ϊ0.15 mol��L-1��min -1
B. T2ʱCH4��ƽ��ת����Ϊ70.0%
C. ���������������䣬T1ʱ��ƽ����ϵ���ٳ���0.30 mol CH4��0.80 mol H2O(g)��ƽ��������Ӧ�����ƶ�
D. ���������������䣬T1ʱ��ƽ����ϵ���ٳ���0.50 mol CH4��1.20 mol NO2����ԭƽ����ȣ�����ƽ��ʱN2��Ũ������
���𰸡�BD
��������A��T1ʱ0��10minCH4��ƽ����Ӧ����Ϊ![]() =0.015molL-1min-1��������֮�ȵ��ڻ�ѧ������֮�ȿ�֪��T1ʱ0��10minNO2��ƽ����Ӧ����Ϊ0.03molL-1min-1����A����B���ɱ������ݿ�֪��T2ʱCH4��ƽ�����ʵ���Ϊ0.15mol����ת���ļ���Ϊ0.50mol-0.15mol=0.35mol����T2ʱCH4��ƽ��ת����Ϊ
=0.015molL-1min-1��������֮�ȵ��ڻ�ѧ������֮�ȿ�֪��T1ʱ0��10minNO2��ƽ����Ӧ����Ϊ0.03molL-1min-1����A����B���ɱ������ݿ�֪��T2ʱCH4��ƽ�����ʵ���Ϊ0.15mol����ת���ļ���Ϊ0.50mol-0.15mol=0.35mol����T2ʱCH4��ƽ��ת����Ϊ![]() ��100%=70.0%����B��ȷ��C��T1ʱ�����Ϊ1L���ɱ��������ݿ�֪��
��100%=70.0%����B��ȷ��C��T1ʱ�����Ϊ1L���ɱ��������ݿ�֪��
CH4(g)+2NO2(g)N2(g)+CO2(g)+2H2O(g)
��ʼ0.51.20 0 0
ת�� 0.4 0.80.40.4 0.8
ƽ�� 0.1 0.40.40.4 0.8
K=![]() =6.4��T1ʱ��ƽ����ϵ���ٳ���0.30molCH4��0.80molH2O(g)��Qc=
=6.4��T1ʱ��ƽ����ϵ���ٳ���0.30molCH4��0.80molH2O(g)��Qc=![]() =25.6��K����ƽ�������ƶ�����C����D��������䣬���ʵ�����Ϊԭ����2������ƽ�ⲻ�ƶ�����ƽ��Ũ�ȱ�Ϊԭƽ���2������ѹǿ����ƽ�������ƶ��������ƽ��ʱN2��Ũ��������ԭƽ��Ũ��С��ԭƽ��Ũ�ȵ�2������D��ȷ����ѡBD��
=25.6��K����ƽ�������ƶ�����C����D��������䣬���ʵ�����Ϊԭ����2������ƽ�ⲻ�ƶ�����ƽ��Ũ�ȱ�Ϊԭƽ���2������ѹǿ����ƽ�������ƶ��������ƽ��ʱN2��Ũ��������ԭƽ��Ũ��С��ԭƽ��Ũ�ȵ�2������D��ȷ����ѡBD��

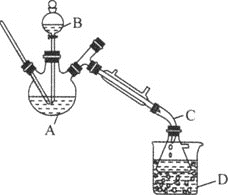
����Ŀ���������л��ϳ��г��õ��ܼ���ijʵ��С����ʵ���������Ҵ���ˮ�Ʊ����ѣ�װ��ʾ��ͼ![]() �гֺͼ���װ������ȥ
�гֺͼ���װ������ȥ![]() ���й����ݺ�ʵ�鲽�����£�
���й����ݺ�ʵ�鲽�����£�
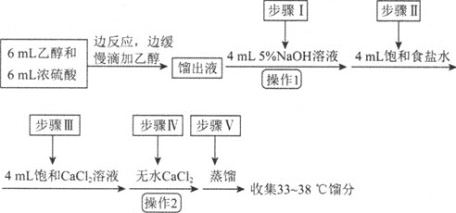
���� | ��Է������� | �ܶ� | �е� | ��ˮ�е��ܽ��� |
�Ҵ� | 46 |
| 78 | ���� |
���� | 74 |
|
| ���� |
��֪������ͬ�����£������ڱ���ʳ��ˮ�б���ˮ�и����ܡ�
���Ȼ��ƿ����Ҵ��γ������![]() ��
��
��ش��������⣺
��1������C������Ϊ_____��
��2�����Ҵ��Ʊ����ѵ��ܷ�ӦΪ![]() ���˷�Ӧ���������У���һ����Ӧ�Ļ�ѧ����ʽΪ
���˷�Ӧ���������У���һ����Ӧ�Ļ�ѧ����ʽΪ![]() ����ڶ�����Ӧ�Ļ�ѧ����ʽΪ_______��
����ڶ�����Ӧ�Ļ�ѧ����ʽΪ_______��
��3����Ӧ�����У�����B��ĩ��Ӧ���뷴ӦҺ�У�ԭ����___��
��4������D��ʢ�б�ˮ����������Ϊ___��
��5������1������Ϊ____������2������Ϊ___��
��6����ʡ�Բ����ᵼ�µĺ����_______��
��7����ʵ������й�����18mL�Ҵ������յõ�8.7g���ѣ������ѵIJ���Ϊ______![]() ��������ȷ��
��������ȷ��![]() ��
��