��Ŀ����
��1����֪���ױȰ����ȶ�����֪��4P(���ף�s��+5O2��g����2P2O5��s�� ��H1��
4P�����ף�s��+5O2��g����2P2O5��s�� ��H2����H1�ͦ�H2�Ĺ�ϵ�ǡ�H1 ��H2���������������
��������
��2����֪H2��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-726.5kJ��mol-1��д����CO2
��H2����Һ̬�״���Һ̬ˮ���Ȼ�ѧ����ʽ ��
��3����֪һ���¶��£����з�Ӧ��ƽ�ⳣ����SO2(g)+1/2O2(g)  SO3(g) K1,CO(g)+1/2O2(g)
SO3(g) K1,CO(g)+1/2O2(g)  CO2(g) K2������ͬ�¶��·�ӦSO2(g)+CO2(g)
CO2(g) K2������ͬ�¶��·�ӦSO2(g)+CO2(g)  SO3(g)+CO(g)��ƽ�ⳣ��Ϊ ��
SO3(g)+CO(g)��ƽ�ⳣ��Ϊ ��
����K1��K2��ʾ��
����6�֣��� ��2��3H2(g)��CO2(g)��CH3OH��l��H2O(l) ��H����130.9 kJ/mol ��3��
���������������1�����ױȰ����ȶ�����˵���������������ڰ�����������������ת��Ϊ���������ȷ�Ӧ����H��0�����ݷ�Ӧ��4P(���ף�s��+5O2��g����2P2O5��s�� ��H1�͢�4P�����ף�s��+5O2��g����2P2O5��s�� ��H2�����ݸ�˹���ɿ�֪���ڣ��ټ��õ���Ӧ4P�����ף�s����4P(���ף�s������÷�Ӧ�ġ�H����H2����H1��0�����ԡ�H1����H2��
��2��H2��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-726.5kJ��mol-1�����з�Ӧ��H2(g)��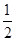 O2(g)��H2O(l) ��H����285.8kJ/mol����CH3OH��l����
O2(g)��H2O(l) ��H����285.8kJ/mol����CH3OH��l����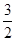 O2(g)��2H2O(l)��CO2(g) ��H����726.5kJ/mol������ݸ�˹���ɿ�֪���١�3���ڼ��õ���Ӧ3H2(g)��CO2(g)��CH3OH��l��H2O(l)����˸÷�Ӧ�ķ�Ӧ�ȡ�H����285.8kJ/mol��3��726.5kJ/mol����130.9 kJ/mol��
O2(g)��2H2O(l)��CO2(g) ��H����726.5kJ/mol������ݸ�˹���ɿ�֪���١�3���ڼ��õ���Ӧ3H2(g)��CO2(g)��CH3OH��l��H2O(l)����˸÷�Ӧ�ķ�Ӧ�ȡ�H����285.8kJ/mol��3��726.5kJ/mol����130.9 kJ/mol��
��3�����ݷ�Ӧ��SO2(g)+1/2O2(g)  SO3(g)����CO(g)+1/2O2(g)
SO3(g)����CO(g)+1/2O2(g) CO2(g)��֪���٣��ڼ��õ���ӦSO2(g)+CO2(g)
CO2(g)��֪���٣��ڼ��õ���ӦSO2(g)+CO2(g)  SO3(g)+CO(g)����������ͬ�¶��·�ӦSO2(g)+CO2(g)
SO3(g)+CO(g)����������ͬ�¶��·�ӦSO2(g)+CO2(g)  SO3(g)+CO(g)ƽ�ⳣ��K��
SO3(g)+CO(g)ƽ�ⳣ��K�� ��
��
���㣺���鷴Ӧ�ȵļ��㡢�Ȼ�ѧ����ʽ����д�Լ�ƽ�ⳣ���ļ����

�����Ѿ�����Ӱ�����ǵ����滷�����������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ��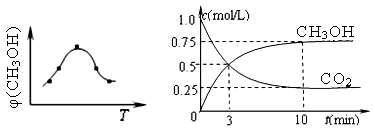
ͼ22-1 ͼ22-2 ͼ22-3
��1�����ü������ԭNOx��
��CH4(g) + 4NO2(g) =" 4NO(g)" + CO2(g) + 2H2O(g) ��H1=-574kJ?mol-1
��CH4(g) + 4NO(g) = 2N2(g) + CO2(g) + 2H2O(g) ��H2=-1160kJ?mol-1
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ ��
��2����CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��
CO2(g) + 3H2(g) = CH3OH(g) + H2O(g) ��H3
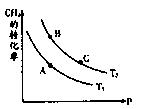
��ȡ��ݵ����CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״������������(CH3OH) �뷴Ӧ�¶�T�Ĺ�ϵ���ߣ���ͼ22-1����������CO2ת��Ϊ�״���Ӧ�ġ�H3 0�����������������=������
����һ���º����ܱ������г���1mol CO2��3mol H2������������Ӧ�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ22-2��ʾ��
����˵����ȷ���� ������ĸ���ţ���
| A����10min������������ٳ���1molCO2��3molH2�����ٴδﵽƽ��ʱc(CH3OH) ="1.5" mol/L |
| B���ﵽƽ��ʱ��������ת����Ϊ0.75 |
| C��0��10�����ڣ�������ƽ����Ӧ����Ϊ0.075mol/��L?min�� |
| D�����¶��£���Ӧ��ƽ�ⳣ����ֵΪ3/16 |
��3��ij���������н���������������һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʡ��������е�SO2��NO2�����ʵ���֮��Ϊ1��1����÷�Ӧ�Ļ�ѧ����Ϊ ��
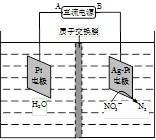
��4���绯ѧ����NO3- ��ԭ������22-3ͼ��ʾ��
�ٵ�Դ����Ϊ (�A����B��)��������ӦʽΪ ��
������������ת����1mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ g��
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ�Ͽ�����ú���������ˮú�����ϳɶ�����( CH3OCH3)����ش��������⣺
(1)����ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2(g)+CO(g)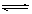 CH3OH(g) ��H= ��90��8kJ/mol
CH3OH(g) ��H= ��90��8kJ/mol
��2CH3OH(g)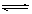 CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
��CO(g)+H2O(g)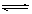 CO2(g)+H2(g) ��H=��41��3kJ/mol
CO2(g)+H2(g) ��H=��41��3kJ/mol
�ܷ�Ӧ��3H2(g)+3CO(g)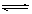 CH3OCH3(g)+CO2(g) �ġ�H= ��
CH3OCH3(g)+CO2(g) �ġ�H= ��
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�ǣ�
________������ĸ���ţ���
a��ѹ����� b��������� c������CO2��Ũ�� d������CO��Ũ��
e������������ѣ�CH3OCH3��
(2)��֪��Ӧ��2CH3OH(g)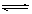 CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ�ȣ�mol��L-1�� | 0��40 | 0��6 | 0��6 |
 _________
_________ ���>������<����=������
���>������<����=�������ڸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK=_____,�¶����ߣ��÷�Ӧ��ƽ�ⳣ��K____�����������С�����䡱��
������һ����ࡢ��Ч��������Դ��
I.�ü�����ȡ�����ķ�Ӧ��Ϊ�������������仯����ͼ��ʾ��
��1�������ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�� ��
II.���ݻ�Ϊ1L���ܱ������ڣ�����0.1molCO��0.1molH2O���ڴ������ڵ������¸��¼���ʹ�䷴Ӧ�����CO2��Ũ����ʱ��仯��ͼ����ͼ��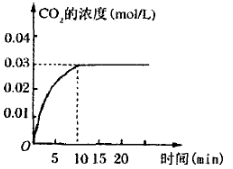
��2���ڸ��¶��£��ӷ�Ӧ��ʼ���ﵽƽ��ʱ��CO��ƽ����Ӧ����Ϊ ��
��3�����¶��£��˷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ�������÷�����ʾ�� ��
��4�����иı��У���ʹƽ��������Ӧ�����ƶ����� ��
| A�������¶� | B������ѹǿ |
| C������H2O��g����Ũ�� | D������CO2��g����Ũ�� |
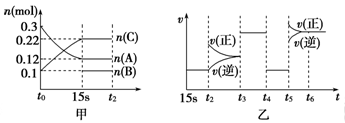
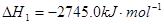


 CO��g��+3H2��g�����Իش��������⡣
CO��g��+3H2��g�����Իش��������⡣