��Ŀ����
�����Ѿ����Ƴ��Ա���Ϊȼ�ϵ�����ȼ�ϵ�أ������Ϊ����̼���Σ�����ܷ�Ӧ����ʽΪ��C3H8+5O2=3CO2+4H2O��
��1����֪��2C3H8��g��+7O2��g��=6CO��g��+8H2O��l��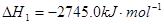
C��s��+O2��g��=CO2��g�� 
2C��s��+O2��g��=2CO��g�� 
��ӦC3H8��g��+5O2��g��=3CO2��g��+4H2O��1���ġ�H___________________��.
��2���õ�ص�����ͨ��O2��CO2������ͨ����飬�������ĵ缫��ӦʽΪ_________________����ع���ʱCO32������_____________����
��3���øõ�ص��1L 1 mol��L��1��AgNO3��Һ���˵��ط�Ӧ�Ļ�ѧ����ʽΪ______________________�����õ������0.005molC3H8ʱ��������Һ��pHΪ__________����Һ����仯���Բ��ƣ�
��1����2221.5kJ��mol��1��2�֣���λ��λд�������֣�
��2��O2+2CO2+4e�� =2CO32����2�֣�����1�֣�
��3��4AgNO3+2H2O 4Ag+O2�� +4HNO3��2�֣���д��Ӧ��������ƽ�����֣� 1��2�֣�
4Ag+O2�� +4HNO3��2�֣���д��Ӧ��������ƽ�����֣� 1��2�֣�
���������������1�����ݷ���ʽ�Ⱥ�˳����ֱ�Ϊ�١��ڡ��ۣ�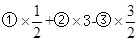 ���õ�����ʽC3H8��g��+5O2��g��=3CO2��g��+4H2O��1������Ӧ��Ҳ������ʽ�����H=
���õ�����ʽC3H8��g��+5O2��g��=3CO2��g��+4H2O��1������Ӧ��Ҳ������ʽ�����H= =��2221.5kJ��mol��1��
=��2221.5kJ��mol��1��
��2��������������Ӧ��O2+2CO2+4e��=2CO32����ԭ��������������ƶ���
��3��������������Ƿ��������ͣ�4AgNO3+2H2O 4Ag+O2�� +4HNO3�����õ������0.005molC3H8ʱ��ת�Ƶ�����0.1mol������0.1molH+��������Ũ��Ϊ0.1mol/L��pHΪ1��
4Ag+O2�� +4HNO3�����õ������0.005molC3H8ʱ��ת�Ƶ�����0.1mol������0.1molH+��������Ũ��Ϊ0.1mol/L��pHΪ1��
���㣺�����Ȼ�ѧ����ʽ����д�����㣬ԭ�������ص�ԭ�������㡣��˹����Ҫ��ʽ������Ӽ�����Ӧ��Ҳ������Ӽ������ڽ��ۺ��⡣

���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ� ũҵ������������������Ҫ���ã� 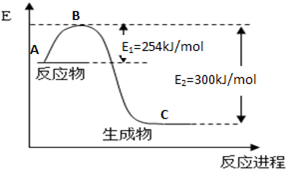
��1����ͼ��N2(g)��H2(g)��Ӧ����1mol NH3(g)�����������仯ʾ��ͼ����д��N2��H2��Ӧ���Ȼ�ѧ����ʽ�� ��
��2������֪�������ݣ�
| ��ѧ�� | H��H | N��N |
| ����/kJ��mol��1 | 435 | 943 |
�Ը��ݱ��м�ͼ�����ݼ���N-H�ļ��� kJ��mol��1��
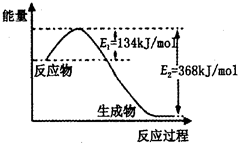
��3���ϳɰ���Ӧͨ��������ý��������ʹ������ý��E1��E2�ı仯�ǣ�E1 ��E2______��
��H (���������С���������䡱)��
��4����NH3����ԭNOX���������������������Ⱦ������
4NH3(g)+3O2(g)�� 2N2(g)+6H2O(g) ����H1��akJ��mol-1
N2(g)+O2(g)��2NO(g)�� ��H2��bkJ/mol
��1mol NH3��ԭNO��N2����÷�Ӧ�����еķ�Ӧ�ȡ�H3�� kJ/mol���ú�a��b��ʽ�ӱ�ʾ����
�������ǡ�����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѡ�
��ش��������⣺
��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ_______________________________________��
��2��ú�����������в������к���������Һ���գ�����������ʽ�Σ��÷�Ӧ��
��ѧ����ʽΪ__________________________________________________________��
��3������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g�� CH3OH��g������H=-90.8kJ��mol��1
CH3OH��g������H=-90.8kJ��mol��1
��2CH3OH��g�� CH3OCH3��g��+H2O��g������H=-23.5kJ��mol��1
CH3OCH3��g��+H2O��g������H=-23.5kJ��mol��1
��CO��g��+H2O��g�� CO2��g��+H2��g������H=-41.3kJ��mol��1
CO2��g��+H2��g������H=-41.3kJ��mol��1
�ܷ�Ӧ��3H2��g��+3CO��g�� CH3OCH3��g��+CO2��g���ġ�H= ��
CH3OCH3��g��+CO2��g���ġ�H= ��
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
________________������ĸ���ţ���
a������b���������c������CO2��Ũ��d������CO��Ũ��e�������������
��4����֪��Ӧ��2CH3OH��g��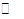 CH3OCH3��g��+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400��
CH3OCH3��g��+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400��
���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L�� | 0.44 | 0.6 | 0.6 |
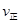 _______
_______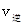 ���>������<����=������
���>������<����=��������������CH3OH��l0min��Ӧ�ﵽƽ�⣬��ʱc(CH3OH)=__________����ʱ
���ڷ�Ӧ����v(CH3OH)=__________________��
 2NH3��g�� ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3��g�� ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���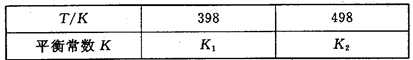
 2H2O��l�� ��H����483��6 kJ��mol
2H2O��l�� ��H����483��6 kJ��mol 2K2CO3��6H2O
2K2CO3��6H2O SO3(g) K1,CO(g)+1/2O2(g)
SO3(g) K1,CO(g)+1/2O2(g) 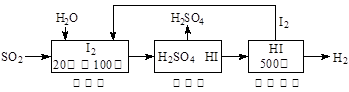
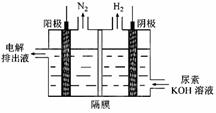
 Ni(OH)2��M
Ni(OH)2��M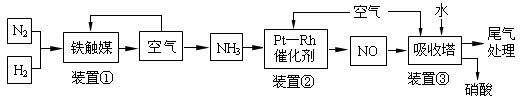
 2NH3(g) ��H����92.4 kJ��mol-1��Ӱ�졣ʵ������ͼ��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����
2NH3(g) ��H����92.4 kJ��mol-1��Ӱ�졣ʵ������ͼ��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����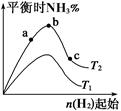
 2NO(g) ��H����180.5 kJ��mol-1
2NO(g) ��H����180.5 kJ��mol-1