��Ŀ����
��14�֣������ת������Դ���úͻ�����������Ҫ�о����⡣�������������ж��ַ�����
��1�����ռ�����H2S�����Һ���뵽����20ͼ��1��ʾ�ĵ��ص����������е�⡣���������������������·�Ӧ��S2����2e�� S ��n��1��S+ S2��
S ��n��1��S+ S2�� Sn2��
Sn2��
��д�����ʱ�����ĵ缫��Ӧʽ�� ��
�ڵ�������������Һ��ϡ�����ữ�õ����ʣ������ӷ���ʽ��д�� ��
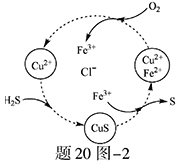
��2����H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת������20ͼ��2��ʾ��
����ͼʾ��ת���У����ϼ۲����Ԫ���� ��
�ڷ�Ӧ�е���1molH2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬��Ҫ����O2�����ʵ���Ϊ ��
�����¶�һ���Ͳ�������Һ�������£�����ͨ�������壬����ֽ��衣��ʹ���ɵ������в���CuS���ɲ�ȡ�Ĵ�ʩ�� ��
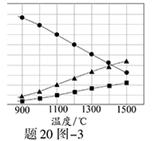
��3��H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ������������20ͼ��3��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ ��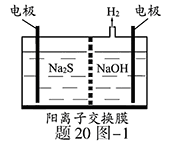
��1���� 2H2O+2e-=H2��+2OH-
�� Sn2-+2H+="(n-1)S��+" H2 S��
��2����Cu��Cl ��0.5mol ����߿����ı���������������ͨ������
��3��2H2S 2H2+S2
2H2+S2
���������������1��������ˮ�����������ӷŵ磻��������Sn2-�����ᷢ���绯��Ӧ��Ӧ�������ʺ����⣻��2�����ݵ����غ㣬������Ӹ��������������õ���Ϊ0.5mol�����������Ӻ�����ʹ��ͭ�������������������������ʹ�������ӱ������������3����ͼ�ж������������������Ϊ2:1��ֻ�ܲ���S2�ŷ������⡣
���㣺���⿼�鳣�����ӵĴ����������⡣

����ɫ��ˮ��Һ���ܴ��������һ���ǣ� ��
| A��Ba2+��AlO2����OH����Al3�� |
| B��K+��Fe3+��MnO4����SCN�� |
| C��Mg2+��H+��SO42����S2O32�� |
| D��NH4+��Ca2+��NO3����Cl�� |
ijͬѧ��һ��ɫ����Һ���з����ó�����Һ�к�������ij�����ӣ�����Ϊ��������Ӧ���ǣ� ��
| A��Al3+��NO3�D��K+��SO42�� | B��Ca2+��H+��CO32����AlO2�D |
| C��OH����SO42����NH4+��Al3+ | D��Fe3+��Mg2+��NO3����Cl�� |
��10�֣����������ҹ���������̼���о����ش��չ���绡���ϳɵ�̼���ܣ������д������ʡ���̼������������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ:
C+ K2Cr2O7 + H2SO4(ϡ) CO2��+ Cr2 (SO4) 3+ + .
CO2��+ Cr2 (SO4) 3+ + .
��1���˷�Ӧ���������� ����������Ԫ���� ��
��2����ɲ���ƽ������Ӧ�Ļ�ѧ����ʽ��
��3��H2SO4��������Ӧ�б��ֳ����������� ����ѡ���ţ�
| A������ | B�������� | C����ˮ�� | D����ˮ�� |
��5��K2Cr2O7�����ڲⶨ�����εĺ���������FeSO4����0.4000�ˣ��ܽ��ữ����Ũ��Ϊ0.02000mol/L��K2Cr2O7����Һ�ζ������ı���Һ20.00mL�����������FeSO4����������Ϊ ��
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | 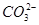 �� ��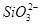 �� ��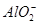 ��Cl�� ��Cl�� |
| ������ | Al3����Cu2����Mg2����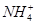 ��Na�� ��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y�����(V)�Ĺ�ϵ��ͼ��ʾ��
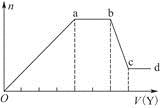
����Y�����ᣬ����Һ�к��еĽ�����������____________________________��
ab�η�����Ӧ�������ӷ���ʽΪ______________________________________��
����Oa����Y��Һ��Ӧ�����ӵ����ʵ���֮��Ϊ__________[Ҫ�������ӷ��ţ���n(Na��)]��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ
_________________________________________________________________��
�����������ӵ�ˮ�����أ�����H����OH����Ӱ�죬����Һ��ֻ����4�����ӣ������ǵ����Ӹ�����Ϊ____________________________________________[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
(2)��Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4����Sn=2Sn2����
2Sn2����O2��4H��=2Sn4����2H2O��
2H����SnO
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�����ӷ���ʽ��
___________________________________��________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������(����ʽ)__________��
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn(OH)2, �ü���__________��
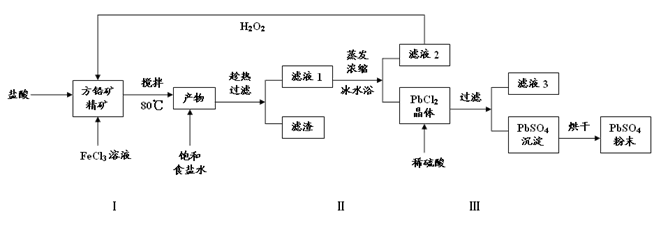
 PbCl4-(aq) ��H��0
PbCl4-(aq) ��H��0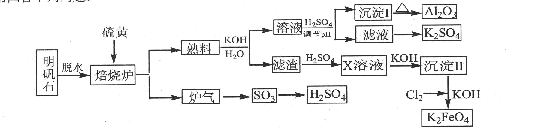
 2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .
2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .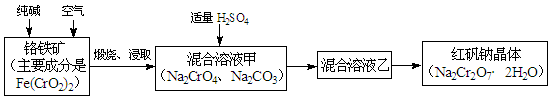
 2CrO42��+2H+����д����ƽ���ƽ�ⳣ������ʽK= ����������ˮϡ�ͣ�ƽ�⽫ �ƶ�(���������������)��
2CrO42��+2H+����д����ƽ���ƽ�ⳣ������ʽK= ����������ˮϡ�ͣ�ƽ�⽫ �ƶ�(���������������)��