��Ŀ����
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | 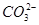 �� ��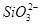 �� ��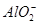 ��Cl�� ��Cl�� |
| ������ | Al3����Cu2����Mg2����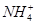 ��Na�� ��Na�� |
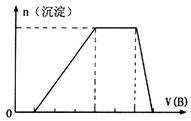
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y�����(V)�Ĺ�ϵ��ͼ��ʾ��
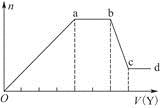
����Y�����ᣬ����Һ�к��еĽ�����������____________________________��
ab�η�����Ӧ�������ӷ���ʽΪ______________________________________��
����Oa����Y��Һ��Ӧ�����ӵ����ʵ���֮��Ϊ__________[Ҫ�������ӷ��ţ���n(Na��)]��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ
_________________________________________________________________��
�����������ӵ�ˮ�����أ�����H����OH����Ӱ�죬����Һ��ֻ����4�����ӣ������ǵ����Ӹ�����Ϊ____________________________________________[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
(2)��Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4����Sn=2Sn2����
2Sn2����O2��4H��=2Sn4����2H2O��
2H����SnO
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�����ӷ���ʽ��
___________________________________��________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������(����ʽ)__________��
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn(OH)2, �ü���__________��
(1)��Na����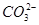 ��2H��=H2O��CO2��
��2H��=H2O��CO2��
n(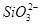 )��n(
)��n(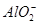 )��11��2
)��11��2
��Al(OH)3��OH��=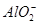 ��2H2O
��2H2O
N(Al3��)��N(Mg2��)��N(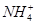 )��N(Cl��)��2��1��4��12
)��N(Cl��)��2��1��4��12
(2)��Sn��2H��=Sn2����H2��
Sn2����Cl2=Sn4����2Cl��
��SnO2����NH3��H2O
����(1)����YΪ���ᣬ��Oa�η�ӦΪ2H����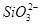 =H2SiO3����H����
=H2SiO3����H����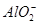 ��H2O=Al(OH)3����ab�η�ӦΪ2H����
��H2O=Al(OH)3����ab�η�ӦΪ2H����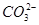 =H2O��CO2����bc�η�ӦΪAl(OH)3��3H��=Al3����3H2O��X��Һ�к���
=H2O��CO2����bc�η�ӦΪAl(OH)3��3H��=Al3����3H2O��X��Һ�к���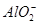 ��
��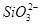 ������������ֻ��Na�����Դ��ڣ���bc�η�Ӧ���ĵ�H�������ʵ���Ϊx�������bc�η�Ӧ�ɵã�n(
������������ֻ��Na�����Դ��ڣ���bc�η�Ӧ���ĵ�H�������ʵ���Ϊx�������bc�η�Ӧ�ɵã�n(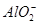 )��
)�� x������Oa�η�Ӧ�ɵã�n(
x������Oa�η�Ӧ�ɵã�n(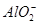 )��2n(
)��2n(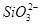 )��4x�����n(
)��4x�����n(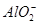 )��n(
)��n(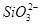 )��2��11������YΪNaOH��Һ����Oa�η�ӦΪAl3����3OH��=Al(OH)3����Mg2����2OH��=Mg(OH)2����ab�η�ӦΪ
)��2��11������YΪNaOH��Һ����Oa�η�ӦΪAl3����3OH��=Al(OH)3����Mg2����2OH��=Mg(OH)2����ab�η�ӦΪ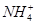 ��OH��
��OH�� NH3��H2O��bc�η�ӦΪAl(OH)3��OH��=
NH3��H2O��bc�η�ӦΪAl(OH)3��OH��=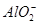 ��2H2O����bc�η�Ӧ���ĵ�OH�������ʵ���Ϊy�������ab�η�Ӧ�ɵ�n(
��2H2O����bc�η�Ӧ���ĵ�OH�������ʵ���Ϊy�������ab�η�Ӧ�ɵ�n(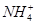 )��2y������bc�η�Ӧ�ɵ�n(
)��2y������bc�η�Ӧ�ɵ�n(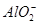 )��y�������Ԫ���غ��֪n(Al3��)��y������Oa�η�Ӧ�ɵ�3n(Al3��)��2n(Mg2��)��4y�����n(Mg2��)��y�����ݵ���غ�ɵ�n(Cl��)��6y���ɵã�n(Al3��)��n(Mg2��)��n(
)��y�������Ԫ���غ��֪n(Al3��)��y������Oa�η�Ӧ�ɵ�3n(Al3��)��2n(Mg2��)��4y�����n(Mg2��)��y�����ݵ���غ�ɵ�n(Cl��)��6y���ɵã�n(Al3��)��n(Mg2��)��n(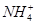 )��n(Cl��)��2��1��4��12��(2)��Sn�ļ�������ΪSn2����Sn4������Ǩ���������ᷴӦ�����������������Ӧ�����ӷ���ʽ����FeCl3��Һ���ɺ�����������ù���ΪFe2O3����SnCl4��Һ���ɺ�����������ù���ӦΪSnO2������2H����SnO
)��n(Cl��)��2��1��4��12��(2)��Sn�ļ�������ΪSn2����Sn4������Ǩ���������ᷴӦ�����������������Ӧ�����ӷ���ʽ����FeCl3��Һ���ɺ�����������ù���ΪFe2O3����SnCl4��Һ���ɺ�����������ù���ӦΪSnO2������2H����SnO Sn(OH)2
Sn(OH)2 Sn2����2OH����֪Sn(OH)2�������ԣ���Ӧ������NH3��H2O��SnCl2��Ӧ��ȡSn(OH)2�Ա�������ǿ�Ӧ��
Sn2����2OH����֪Sn(OH)2�������ԣ���Ӧ������NH3��H2O��SnCl2��Ӧ��ȡSn(OH)2�Ա�������ǿ�Ӧ��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д����и������ʵ�ϡ��Һ���Ӧ��������ǰ�ߵ�����ߣ����Ǻ��ߵ���ǰ�ߣ���Ӧ������ͬ����
| A��Na2CO3��HCl | B��NaHCO3��Ba(OH)2 | C��NaAlO2��H2SO4 | D��AgNO3��NH3��H2O |
�о���Ա�������õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)�������᳧��β��SO2���Ʊ������̵������������£�
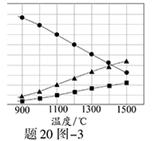
��֪������Һ��pH��2�����еĽ���������Ҫ��Mn2����������������Fe2����Al3����Ca2����Pb2���������������ӡ�PbO2�������Դ���MnO2��PbSO4��һ�������ʡ��йؽ������ӵİ뾶���γ������������ʱ��pH���±��������������������������ӵ�Ч������ͼ��
| ���� | ���Ӱ뾶(pm) | ��ʼ���� ʱ��pH | ��ȫ���� ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |

��ش��������⣺
��1��д����������������Mn2+��Ӧ�Ļ�ѧ����ʽ ��
��2��������������Ҫ��Ӧ�����ӷ���ʽ ��
��3�����������Һ���м���ʯ�ҽ������ڵ���pHֵ���˴�����pHֵ�õ��������� ��Ӧ����pH�ķ�ΧΪ ��
��4�������������������ڳ�ȥ���ʽ������ӡ�������ͼ������Ϣ�ش𣬾�������������������Ч���������� �� �ȣ����������ȥ����Ҫ����Ϊ�� ��
��5��CaSO4��һ�������ʣ���֪Ksp(CaSO4)=9.10��10��6���ֽ�c mol��L��1CaCl2��Һ��2.00��10��2 mol��L��1Na2SO4��Һ�������ϣ���������ı仯���������ɳ���ʱ��c����Сֵ������������
����ѧ����ѡ��2����ѧ�뼼������15�֣�
��Ƴ���ͭ��ˮ�к���CN-��Cr2O72-���ӣ���Ҫ������������ŷš��ó��ⶨ�������̽��з�ˮ�������ش��������⣺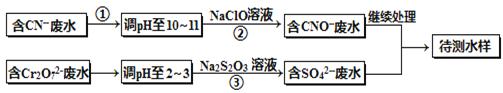
��1������������ˮ��������Ҫʹ�õķ�����_________________��
��2�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ______________��
��3��������У�ÿ����0.4mol Cr2O72-ʱת�Ƶ���2.4mol���÷�Ӧ�����ӷ���ʽΪ ��
��4��ȡ��������ˮ�����Թ��У�����NaOH��Һ���۲쵽����ɫ�������ɣ��ټ�Na2S��Һ����ɫ����ת���ɺ�ɫ��������ʹ�û�ѧ��������ֽ��Ͳ����������ԭ�� ��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4 ��7H2O��Cr2O72-��ԭ��Cr3+������pH��Fe��Crת�����൱�ڣ� ����������,�������ֱ�ʾԪ�ؼ�̬���ij�����
����������,�������ֱ�ʾԪ�ؼ�̬���ij�����
����1mol Cr2O72-�������a mol FeSO4 ? 7H2O�����н�����ȷ����_______��
| A��x ="0.5" ,a ="8" | B��x ="0.5" ,a =" 10" | C��x =" 1.5" ,a =8 | D��x =" 1.5" ,a = 10 |
 S ��n��1��S+ S2��
S ��n��1��S+ S2��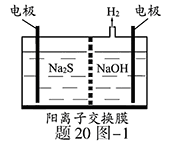
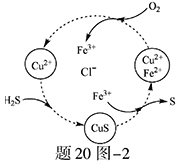


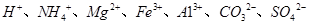 �е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��
�е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��