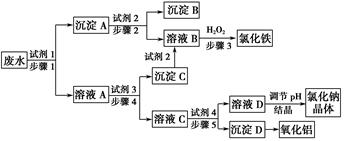
��Ŀ����
�����Ĺ�ҵ��ˮ������ֱ�������Ӻ������ŷţ������������ع�����ij�ӵ������мס��������������ų��Ĺ�ҵ��ˮ������K����Fe3����Ba2����Ag����Cl����SO42����NO3����OH���е����֡���֪�׳���ˮpH>7��
(1)�׳���ˮ�к��е��������ӿ����ǣ�_______________________________��
(2)�ҳ���ˮ�к��е��������ӿ����ǣ�______________________________��
(3)���ҳ���ˮ���д���������֮һ��������ˮ�м���һ������________(ѡ��
������̼�������������������ۡ�)�����Ի������еĽ���__________(��
Ԫ�ط���)��
(4)������Ⱦ����һ����Ҫ��ʩ�ǽ��ס��������ķ�ˮ��������ϣ���ʹ��ˮ�е�________(�����ӷ���)ת��Ϊ�������Ӷ������Ⱦ�̶ȡ����˺����÷�ˮ��Ҫ��________�������ڽ���ũ�
(1)OH����Cl����SO42����K��
(2)Fe3����Ba2����Ag����NO3��
(3)���ۡ�Ag
(4)OH����Fe3����Cl����Ag����SO42����Ba2����KNO3
����

��������(ClO2)Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
(1)��ҵ���Ʊ�ClO2�ķ�Ӧԭ��Ϊ2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
��Ũ�����ڷ�Ӧ����ʾ������������________��
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� |
| C��ֻ�������� | D�������Ժ����� |
(2)Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
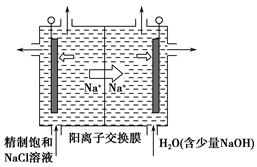
����ͼ����ʯī���缫��һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��ʾ��ͼ������������ClO2�ĵ缫��ӦʽΪ_____________________________________��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL(��״��)ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ________mol����ƽ���ƶ�ԭ������������pH�����ԭ��______________________________________��
(3)ClO2����ˮ��Fe2����Mn2����S2����CN���������Ե�ȥ��Ч����ij������ˮ�к�CN��a mg/L������ClO2��CN���������������������������ɣ������ӷ�Ӧ����ʽΪ________________������100 m3������ˮ��������ҪClO2________mol��
 S ��n��1��S+ S2��
S ��n��1��S+ S2��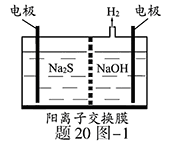
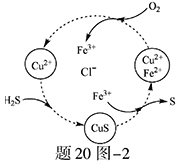
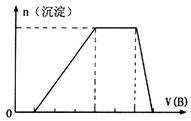
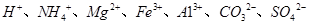 �е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��
�е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��