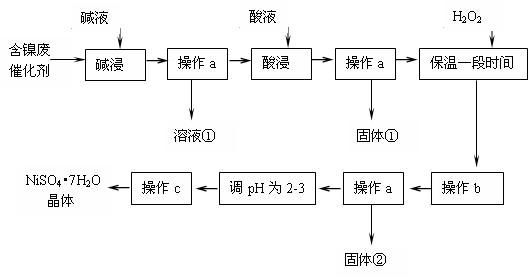
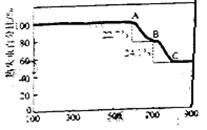
��Ŀ����
ij�༶��̽��С���о�����ͭ�ܷ�������ص����ȷֽ������������ã����������ʵ�飺
��һ�� �Ʊ�����ͭ
��1����ȡ5.0g CuSO4��5H2O���壬��ϸ���ܽ⣺�˲�����Ҫ�������� ��
��2����ʢ������ͭ��Һ���ձ��еμ�NaOH��Һ��ֱ�����ٲ�������Ϊֹ���˲�ʵ������ ��
���ӷ���ʽΪ�� ��
��3�� A���Ѳ��裨2���е���Һ�ͳ���ת�Ƶ��������ڣ������������ڣ����裬ֱ������ȫ����Ϊ��ɫ���壬ֹͣ���ȣ��ٹ��ˡ�ϴ�ӡ������ת�Ƶ��в�����ϸ���á�
B���Ѳ��裨2���е���Һ�ͳ���ת�Ƶ��������й��ˣ�ϴ�ӣ��������������������н�����������ȫ����Ϊ��ɫ����Ϊֹ����ת�Ƶ��в�����ϸ���á�
����Ϊ��һ��������ã�˵�����ɣ� ��
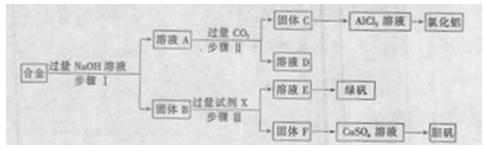
������֤������ͭ���������
��ƶԱ�ʵ�����ȡ��������أ��ֱ������֧�Թܣ�������һ���м��뾭��ȷ������ng����ͭ��ĩ���ڽ����������ͬʱ����ͬ��������ȷ���ȣ����ų�������ͨ��ˮ�У���ֹͣ���ȣ���ȴ����ԭ�Ȼ�������ͭ�ķ�Ӧ�������ˮ�ܽ⣬С�Ĺ��ˣ��õ��˳��ϴ�Ӳ�����ܹ۲��˳������ɫ��״̬���ݽ��˳����̿�ۻ�ͣ����ܱ������и��¼��ȣ�����Ӧ����������ͨ������ʯ��ˮ�У����۲���������
��ش��������⣺
��1������ͭ�����������������ǣ� ��
��2������ʵ�鲽�裬��һ���������ܣ���ָ������һ���� �������ţ����ò���Ӧ�����θĽ��� ��
��3��Ҫ�ﵽʵ��Ŀ�ģ���Ӧ��������һ��ʵ�鲽�裬���ʵ�鲽���� ��
��4��������һ��ʵ��װ�úͲ����滻�ڢݲ�ʵ�飬Ҳ���Դﵽ��һ����ʵ��Ŀ�ģ����ͼ��������������������ѡ���������ӳ�һ�����������Ҫ�������װ�á�
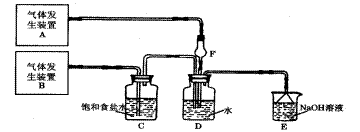
���ʵ��װ�ð������������ҵ�����˳���� �� �� �� �� ��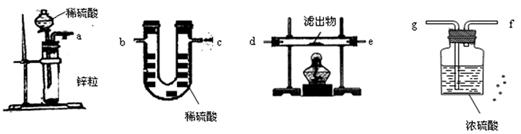
��һ�� �Ʊ�����ͭ
��1����ȡ5.0g CuSO4��5H2O���壬��ϸ���ܽ⣺�˲�����Ҫ�������� ��
��2����ʢ������ͭ��Һ���ձ��еμ�NaOH��Һ��ֱ�����ٲ�������Ϊֹ���˲�ʵ������ ��
���ӷ���ʽΪ�� ��
��3�� A���Ѳ��裨2���е���Һ�ͳ���ת�Ƶ��������ڣ������������ڣ����裬ֱ������ȫ����Ϊ��ɫ���壬ֹͣ���ȣ��ٹ��ˡ�ϴ�ӡ������ת�Ƶ��в�����ϸ���á�
B���Ѳ��裨2���е���Һ�ͳ���ת�Ƶ��������й��ˣ�ϴ�ӣ��������������������н�����������ȫ����Ϊ��ɫ����Ϊֹ����ת�Ƶ��в�����ϸ���á�
����Ϊ��һ��������ã�˵�����ɣ� ��
������֤������ͭ���������
��ƶԱ�ʵ�����ȡ��������أ��ֱ������֧�Թܣ�������һ���м��뾭��ȷ������ng����ͭ��ĩ���ڽ����������ͬʱ����ͬ��������ȷ���ȣ����ų�������ͨ��ˮ�У���ֹͣ���ȣ���ȴ����ԭ�Ȼ�������ͭ�ķ�Ӧ�������ˮ�ܽ⣬С�Ĺ��ˣ��õ��˳��ϴ�Ӳ�����ܹ۲��˳������ɫ��״̬���ݽ��˳����̿�ۻ�ͣ����ܱ������и��¼��ȣ�����Ӧ����������ͨ������ʯ��ˮ�У����۲���������
��ش��������⣺
��1������ͭ�����������������ǣ� ��
��2������ʵ�鲽�裬��һ���������ܣ���ָ������һ���� �������ţ����ò���Ӧ�����θĽ��� ��
��3��Ҫ�ﵽʵ��Ŀ�ģ���Ӧ��������һ��ʵ�鲽�裬���ʵ�鲽���� ��
��4��������һ��ʵ��װ�úͲ����滻�ڢݲ�ʵ�飬Ҳ���Դﵽ��һ����ʵ��Ŀ�ģ����ͼ��������������������ѡ���������ӳ�һ�����������Ҫ�������װ�á�
���ʵ��װ�ð������������ҵ�����˳���� �� �� �� �� ��

��һ����1��������ƽ���в����ձ�����������
��2��������ɫ��״���� Cu2++2OH-=Cu(OH)2��
��3��A������ã���Ϊ����ͭ���������ٶȿ졣
(��)��1���ӿ췴Ӧ���ʣ��ҷ�Ӧǰ�����������ʲ��䡣
��2��ȷ���������������������
��3�������������˳����������
��4��a �� g �� f �� d ����e���� e����d���� b

(��c)
��2��������ɫ��״���� Cu2++2OH-=Cu(OH)2��
��3��A������ã���Ϊ����ͭ���������ٶȿ졣
(��)��1���ӿ췴Ӧ���ʣ��ҷ�Ӧǰ�����������ʲ��䡣
��2��ȷ���������������������
��3�������������˳����������
��4��a �� g �� f �� d ����e���� e����d���� b


|
|
��

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ

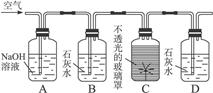
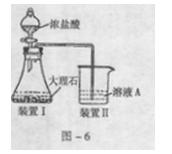
 ��1��ʵ��ʱ�����ܽӿڵ�˳���� ��
��1��ʵ��ʱ�����ܽӿڵ�˳���� �� ����4��֤����������к���CO��ʵ�������� ��
����4��֤����������к���CO��ʵ�������� ��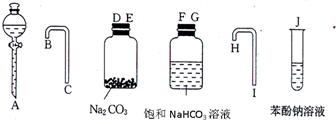
 ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ
����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺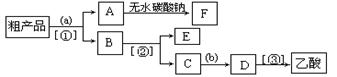
 ��
�� ��Һ�����뷽�����������뷽�����Ƿ�Һ
��Һ�����뷽�����������뷽�����Ƿ�Һ ��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�
��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�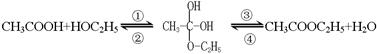
 ����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��
����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��