��Ŀ����
��14�֣�Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ���������ͼ��ʾ��ʵ��װ�á�����ա�
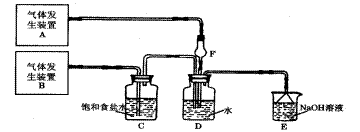
��1��Dװ������Ҫ��Ӧ�����ӷ���ʽΪ ��
F�����������ǣ� ��
��2��Ϊ��֤ͨ��Dװ����������Cl2����SO2��������ȤС���ͬѧ���������Լ���
a �Ȼ���ϡ��Һ b �Ȼ�������Һ c���軯����Һ
d ������Һ e Ʒ����Һ f ���Ը��������Һ
����Cl2������ȡʢ�� ��ѡ��һ�����ţ��Լ����Թܣ� ���� ��ѡ
��һ�����ţ��Լ�������������ȡ����D����Һ�μ����Թ��У�����������
�ǣ� ��
�� ��ѡ�������Լ��е�һ�ֽ�ϱ�Ҫ�IJ����Ϳ�����֤ͨ��Cװ����������Cl2����
����SO2���������Լ���______��ѡ��һ�����ţ�����Ҫ�����ͽ���______ ____��

��1��Dװ������Ҫ��Ӧ�����ӷ���ʽΪ ��
F�����������ǣ� ��
��2��Ϊ��֤ͨ��Dװ����������Cl2����SO2��������ȤС���ͬѧ���������Լ���
a �Ȼ���ϡ��Һ b �Ȼ�������Һ c���軯����Һ
d ������Һ e Ʒ����Һ f ���Ը��������Һ
����Cl2������ȡʢ�� ��ѡ��һ�����ţ��Լ����Թܣ� ���� ��ѡ
��һ�����ţ��Լ�������������ȡ����D����Һ�μ����Թ��У�����������
�ǣ� ��
�� ��ѡ�������Լ��е�һ�ֽ�ϱ�Ҫ�IJ����Ϳ�����֤ͨ��Cװ����������Cl2����
����SO2���������Լ���______��ѡ��һ�����ţ�����Ҫ�����ͽ���______ ____��
��14�֣���ÿ��2�֣�
��1��Cl2+SO2+2H2O��4H++2Cl�D+SO42�D��������
��2���� b��c����d������Һ�ʺ�ɫ������ɫ��
��e Ʒ����ɫ�����Ȳ���ԭ��˵������������Ʒ����ɫ�������ָ�ԭ��˵���������������
��1��Cl2+SO2+2H2O��4H++2Cl�D+SO42�D��������
��2���� b��c����d������Һ�ʺ�ɫ������ɫ��
��e Ʒ����ɫ�����Ȳ���ԭ��˵������������Ʒ����ɫ�������ָ�ԭ��˵���������������
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
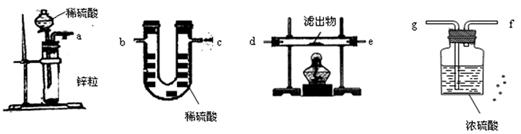
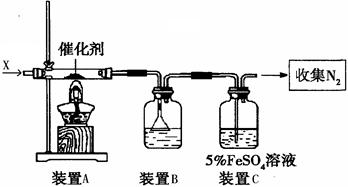
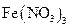 ��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽����
��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽����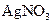 ��Һ���Թܣ��ߵ���2%�İ�ˮ��������ij���ǡ���ܽ�Ϊֹ
��Һ���Թܣ��ߵ���2%�İ�ˮ��������ij���ǡ���ܽ�Ϊֹ ���������ԣ�������Ag��
���������ԣ�������Ag�� ������Ag��
������Ag�� ����֤�˼���1�ij�������д��
����֤�˼���1�ij�������д��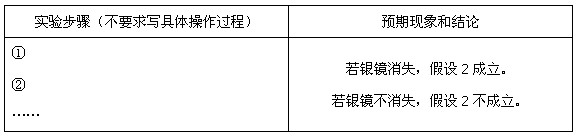
 2Cu2O+O2��
2Cu2O+O2��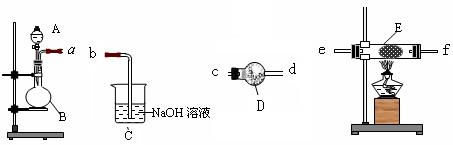
 4NO2(g)����H>0���±�Ϊ��Ӧ��ij�¶��µIJ���ʵ������
4NO2(g)����H>0���±�Ϊ��Ӧ��ij�¶��µIJ���ʵ������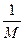 g
g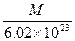 g
g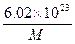 g
g