��Ŀ����
��16�֣�ijͬѧΪ���о���������ʣ�����������ʵ�飺
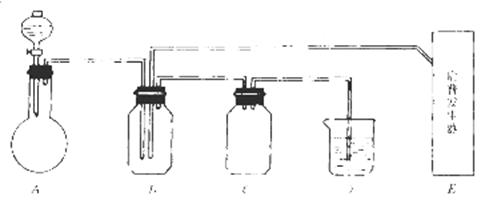
��һ����ͬѧ������ͼ��ʾ��������ҩƷ�������һ����һ�������ʵ��װ�ã�����֤���ᡢ̼��ͱ�����Һ����ǿ����
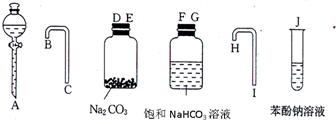
��1��ʵ��װ�õ�����˳���ǣ�A�� �� �� �� C �� �� �� �� ��J
��2��д��ʵ������˵��̼��ȱ��ӵ�����ǿ�Ļ�ѧ����ʽ�� ��
������Ϊ���о������������Ӧ����ͬѧ����������ʵ�飬�������£��� ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ
����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
��1����Ӧ��Ũ������__________������ˮ��������ˮ������
��2�����������ʵ�鲽���Ϊ��������ƿ���ȼ����Ҵ���Ũ���ᣬȻ��ͨ����Һ©���ߵμӴ��ᣬ��������������������������IJ��ʣ���ԭ����_________________��
��3��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������Ҫ��ͼ��Բ�����������ʵ��Լ����ڷ������������ʵ����뷽����
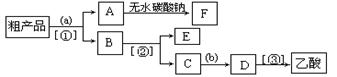
�����Լ��ͷ��뷽����ȷ���� ��
��
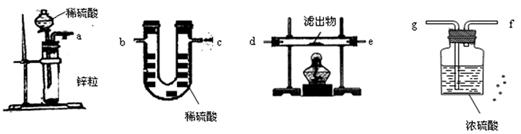
A���Լ�a������������Һ���Լ�b��ϡ������뷽�����������뷽�����Ƿ�Һ�����뷽����������
B���Լ�a�DZ���̼������Һ���Լ�b��ϡ������뷽�����Ƿ�Һ�����뷽�����������뷽����������
C���Լ�a�DZ���̼������Һ���Լ�b��ϡ������뷽�����ǹ��ˣ����뷽�����Ƿ�Һ�����뷽����������
D���Լ�a������������Һ���Լ�b��������뷽������ ��Һ�����뷽�����������뷽�����Ƿ�Һ
��Һ�����뷽�����������뷽�����Ƿ�Һ
��4����ͬѧ�ڲ�������ʱ���� ��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�
��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�
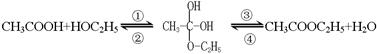
a������Ϊ�������ڼӳɷ�Ӧ���� ���Ӧ��ţ��ڢ٢ڢۢ���ѡȡ��
b�����л�����ʹ���������Ӧ�У���ˮʱ�������� ����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��
����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��
A��18Oֻ�ܱ���ڴ��ǻ���
B�� 18Oֻ�ܱ����������ǻ���
C��18O���Ա���ڴ��ǻ��ϣ�Ҳ���Ա����������ǻ���
D��18O���Ա���ڴ��ǻ��ϣ�Ҳ���Ա����������ǻ��ϣ������Ա����������ʻ�
��
��һ����ͬѧ������ͼ��ʾ��������ҩƷ�������һ����һ�������ʵ��װ�ã�����֤���ᡢ̼��ͱ�����Һ����ǿ����

��1��ʵ��װ�õ�����˳���ǣ�A�� �� �� �� C �� �� �� �� ��J
��2��д��ʵ������˵��̼��ȱ��ӵ�����ǿ�Ļ�ѧ����ʽ�� ��
������Ϊ���о������������Ӧ����ͬѧ����������ʵ�飬�������£���
 ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ
����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺��1����Ӧ��Ũ������__________������ˮ��������ˮ������
��2�����������ʵ�鲽���Ϊ��������ƿ���ȼ����Ҵ���Ũ���ᣬȻ��ͨ����Һ©���ߵμӴ��ᣬ��������������������������IJ��ʣ���ԭ����_________________��
��3��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������Ҫ��ͼ��Բ�����������ʵ��Լ����ڷ������������ʵ����뷽����

�����Լ��ͷ��뷽����ȷ����
 ��
��A���Լ�a������������Һ���Լ�b��ϡ������뷽�����������뷽�����Ƿ�Һ�����뷽����������
B���Լ�a�DZ���̼������Һ���Լ�b��ϡ������뷽�����Ƿ�Һ�����뷽�����������뷽����������
C���Լ�a�DZ���̼������Һ���Լ�b��ϡ������뷽�����ǹ��ˣ����뷽�����Ƿ�Һ�����뷽����������
D���Լ�a������������Һ���Լ�b��������뷽������
 ��Һ�����뷽�����������뷽�����Ƿ�Һ
��Һ�����뷽�����������뷽�����Ƿ�Һ��4����ͬѧ�ڲ�������ʱ����
 ��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�
��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�
a������Ϊ�������ڼӳɷ�Ӧ���� ���Ӧ��ţ��ڢ٢ڢۢ���ѡȡ��
b�����л�����ʹ���������Ӧ�У���ˮʱ��������
 ����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��
����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��A��18Oֻ�ܱ���ڴ��ǻ���
B�� 18Oֻ�ܱ����������ǻ���
C��18O���Ա���ڴ��ǻ��ϣ�Ҳ���Ա����������ǻ���
D��18O���Ա���ڴ��ǻ��ϣ�Ҳ���Ա����������ǻ��ϣ������Ա����������ʻ�
��
��һ����1��A�� D �� E �� B �� C �� F �� G �� H �� I ��J
��2�֣���һ���ͼ�0�֣�
��2��CO2+H2O+C6H5ONa �� C6H5OH+NaHCO3 ��2�֣�
��������1������(2��) ��2����ʱ�����������������������Ӧ���������ķ�
����У�2�֣� ��3��B ��2�֣���4��a���٢� ��2�֣� b���ǣ�2�֣�
A ��2�֣�
��2�֣���һ���ͼ�0�֣�
��2��CO2+H2O+C6H5ONa �� C6H5OH+NaHCO3 ��2�֣�
��������1������(2��) ��2����ʱ�����������������������Ӧ���������ķ�
����У�2�֣� ��3��B ��2�֣���4��a���٢� ��2�֣� b���ǣ�2�֣�
A ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
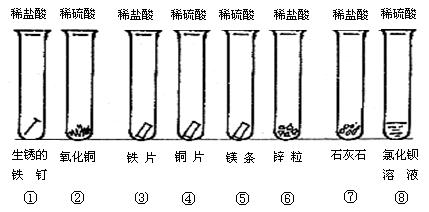
 2Cu2O+O2��
2Cu2O+O2��