��Ŀ����
����Ŀ����1��һ��������ij����H2�����ʵ���֮��1:2�ӳ�����C2H5CH (CH3)2��������Ľṹ��ʽΪ(��д1��)______________________��
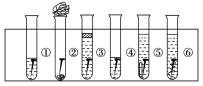
��2������ʽΪC4H8�������˴Ź�������ͼ�����������շ壬�������Ϊ3:1����д������������2�����ʵĽṹ��ʽ___________________��_____________________��
��3���л���Ľṹ��ʽ���£�����ͨ����ͬ�Ļ�ѧ��Ӧ�ֱ��Ƶýṹ��ʽΪ�ҡ��������ʡ�

��ش��������⣺
���ڼס������������У���Ϊͬ���칹�����_________________�����ţ���ͬ�����ɿ����������___________________��
��д���ɼ����ɸ߷��ӻ�����ķ���ʽ��_______________________________________
���𰸡�![]() (��
(��![]() )
) ![]()
![]() ������ �졢������ n
������ �졢������ n![]()
![]()
 +(n-1)H2O
+(n-1)H2O
��������
(1)ij����H2�����ʵ���֮��1��2�ӳɣ�������к���1��̼̼����������̼̼˫�����ݴ�д���������ܵĽṹ��ʽ��
(2)�������˴Ź�������ͼ�����������շ壬�������Ϊ3��1���жϺ��е�Ч��ԭ�����༰��Ŀ���ٽ�Ϸ���ʽC4H8д�����������Ľṹ��ʽ��
(3)�ٸ���ͬ���칹��ĸ�������жϣ��������Ľṹ���������жϣ��ڼ����Ȼ����ǻ����ɷ������۷�Ӧ���ɸ߷��ӻ�����ݴ���д��Ӧ�ķ���ʽ��
(1)һ��������ij����H2�����ʵ���֮��1��2�ӳ�����CH3CH2CH(CH3)2���������2��̼̼˫����1��̼̼����������ܵĽṹ��ʽΪ��![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() (��
(��![]() )��
)��
(2)����ʽΪC4H8�������˴Ź�������ͼ�����������շ壬�������Ϊ3��1��˵�������д�������Hԭ�ӣ��Ҹ�����Ϊ3��1������ܵĽṹ��ʽΪ��![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
(3)���ڼס������������У�������ķ���ʽ��ͬ�����ṹ��ͬ����Ϊͬ���칹�壻�졢�����������������ɿ������࣬�ʴ�Ϊ�������죻�졢��������
��![]() �к����ǻ����Ȼ������Է������۷�Ӧ����Ӧ�ķ���ʽΪ��n
�к����ǻ����Ȼ������Է������۷�Ӧ����Ӧ�ķ���ʽΪ��n![]()
![]()
 +(n-1)H2O���ʴ�Ϊ��n
+(n-1)H2O���ʴ�Ϊ��n![]()
![]()
 +(n-1)H2O��
+(n-1)H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL������ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
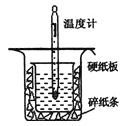
��1����ͼ��ʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��___��
��2��Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������___��
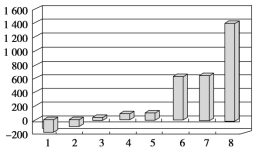
��3��������60mL0.25mol��L-1H2SO4��50mL0.55mol��L-1NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������___����������������������������ʵ���������ȷ���������к���___������������������������
��4��50mL0.55mol/LNaOH��Һ��50mL0.25mol/L������Һ��ʵ���������±���
����д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2-t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ��___ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ50mL0.55mol/LNaOH��Һ��50mL0.25mol/L������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c=4.18J/(g����)�����к�����H=___J/mol(ȡС�����һλ)��
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ�������___(����ĸ)��
a.ʵ��װ�ñ��¡�����Ч����
b.��ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
����Ŀ��Ԫ�����ڱ��е������ڰ���Na��Mg��Al��Si��P��S��Cl��Ar 8��Ԫ�ء���ش��������⣺
��1��SCl2�����е�����ԭ���ӻ����������_______���÷��ӹ���Ϊ_________��
��2����������8��Ԫ�ذ������۵�(��)��С˳����Ƶ�����ͼ(��֪������1������Ar)��ͼ��ʾ����������2��ԭ�ӵĽṹʾ��ͼΪ____________����8��ԭ�ӵĵ����Ų�ʽΪ________________��
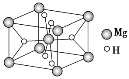
��3���⻯þ������ϵľ����ṹ��ͼ��ʾ����֪�þ�����ܶ�Ϊ�� g��cm��3����þ���Ļ�ѧʽΪ__________�����������Ϊ__________cm3(������NA��ʾ������NA��ʾ�����ӵ�������ֵ)��
��4��ʵ��֤����KCl��MgO��CaO���־���Ľṹ��NaCl����Ľṹ���ƣ���֪NaCl��KCl��CaO����ľ������������±���
���� | NaCl | KCl | CaO |
������/(kJ��mol��1) | 786 | 715 | 3401 |
��KCl��MgO��CaO���־�����۵�Ӹߵ��͵�˳����________________������MgO������һ��Mg2����Χ��������ҵȾ����Mg2����________����
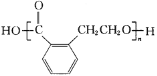
��5�����(![]() )�㷺Ӧ���ںϳ�ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ��
)�㷺Ӧ���ںϳ�ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ��
����Է����к���_______�������������еĴ��������÷���![]() ��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ
��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ![]() )������Է����еĴ�����Ӧ��ʾΪ______________��
)������Է����еĴ�����Ӧ��ʾΪ______________��
����Եķе�Ϊ84�棬����(![]() )�ķе���129~131��֮�䣬���߷е�ϸߣ���ԭ����__________________________________��
)�ķе���129~131��֮�䣬���߷е�ϸߣ���ԭ����__________________________________��
��6��Si��C��O�ijɼ�������£�
��ѧ�� | C��O | C===O | Si��O | Si===O |
360 | 803 | 464 | 640 |
C��O֮�����γɺ���˫����CO2���Ӿ��壬��Si��O֮�������γɺ��е�����SiO2ԭ�Ӿ��壬�������ݷ�����ԭ��_______________________________________________________