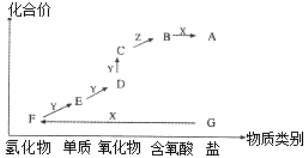
��Ŀ����
����Ŀ����1��25��ʱ����ͬ���ʵ���Ũ�ȵ�������Һ�У���NaCl��NaOH��H2SO4��(NH4)2SO4������ˮ�ĵ���̶��ɴ�С˳��___(�����)��
��2��25��ʱ�������ͬ��Ũ�Ⱦ�Ϊ0.2mol��L-1�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ___�������£�pH=12��NaOH��Һ��pH=2�����ᣬ���������Ϻ���Һ��pHΪ___��
��3�������ӷ���ʽ����̼������Һ�ʼ��Ե�ԭ��___��
��4����֤��CH3COOH���������ʵ��___��
A.CH3COOH�ӷ�
B.�����£�0.1mol��L-1CH3COONa��Һ��pH����7
C.�����ʴ�·�
D.pH=3��CH3COOH��Һϡ��10������ҺpH<4
E.�������Ũ�ȵ�CH3COOH��Һ�����ᣬ�ֱ�������þ�۷�Ӧ����������һ����
F.��һ������CH3COOH��Һ����Na2CO3��Һ�У������ݲ���
���𰸡��ܣ��٣��ڣ��� m<n 7 CO32-+H2O![]() HCO3-+OH- BD
HCO3-+OH- BD
��������
(1)�������ˮ���룬�ε�ˮ��ٽ�ˮ�ĵ��룻
(2)ǿ����ȫ���룬�����ȫ���룬��Ũ�ȵİ�ˮ��NaOH��Һϡ����ͬ����ʱ��ǰ������������Ũ�Ƚϴ�
NaOH��c(OH-)����������c(H+)�������ȣ���Ӧ����Һ�����ԣ�
(3)Na2CO3��ǿ�������Σ�ˮ��ʼ��ԣ�
(4)����������ʵĵ����Dz���ȫ�ģ�����ܽ��Լ����ͨ����ȷ�������ij̶ȣ����Ƚ����Ũ���������c(H+)���ж���ĵ���̶ȡ�
(1)�������ˮ���룬�ʢ�NaOH�͢�H2SO4��Һ��ˮ�ĵ���̶Ƚ�С�����������Ƕ�Ԫǿ�ᣬ����������һԪǿ�����ͬ���ʵ���Ũ�ȵ�������ˮ�ĵ���̶�С������������ˮ�ĵ���̶ȣ���ˮ����δٽ�ˮ�⣬�ʢ�(NH4)2SO4��Һ��ˮ�ĵ���̶����ˮ�����NaCl��ˮ�ĵ�����Ӱ�죬
��ˮ�ĵ���̶ȵ���ȷ����˳��Ϊ����>��>��>�ۣ�
(2) Ũ�Ⱦ�Ϊ0.2mol��L-1�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��10�������ߵ�Ũ������ͬ������NH3��H2O��������ʣ�NaOH��ǿ����ʣ�NaOH�ĵ���̶ȴ���NH3��H2O�ĵ���̶ȣ����NaOH�е�����������Ũ�ȴ���NH3��H2O�е�����������Ũ�ȣ������m<n��
�����£�pH=12��NaOH��c(OH-)=0.01mol/L��pH=2��������c(H+)=0.01mol/L���������Ϻ���ȫ�к�����ǿ��ǿ���Σ�����Һ�����ԣ�pH=7��
(3)Na2CO3��ǿ�������Σ�ˮ��ʼ��ԣ�CO32-+H2O![]() HCO3-+OH-��
HCO3-+OH-��
(4) A.CH3COOH�ӷ������������ʣ�����֤�����Ե�ǿ����ѡ��A����
B.�����£�0.1mol��L-1CH3COONa��Һ��pH����7��˵��CH3COONaΪǿ��������ˮ��ʼ��ԣ���CH3COOHΪ���ᣬѡ��B��ȷ��
C.�����ʴ�·�������֤�����Ե�ǿ����ѡ��C����
D.pH=3��CH3COOH��Һϡ��10������ҺpH<4��˵�������ȫ���룬���ڵ���ƽ�⣬ѡ��D��ȷ��
E.�������Ũ�ȵ�CH3COOH��Һ�����ᣬ�����ʵ�����ͬ���ֱ�������þ�۷�Ӧ����������һ���࣬����˵�����������ǿ����ѡ��E����
F.��һ������CH3COOH��Һ����Na2CO3��Һ�У������ݲ�����˵������������ԣ�������˵��������ǿ����ѡ��F����
��ѡBD��