��Ŀ����
����Ŀ�����ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ�������
��1��T1��ʱ����2L�ܱ������г���0.6molSO3��ͼ1��ʾSO3���ʵ�����ʱ��ı仯���ߡ�

��ƽ��ʱ��SO3��ת����Ϊ______������һλС��������T1���·�Ӧ2SO2(g)+O2(g) ![]() 2SO3(g) ��ƽ�ⳣ��Ϊ____________���������������䣬��8minʱѹ�������������1L����n(SO3)�ı仯����Ϊ_______������ĸ����
2SO3(g) ��ƽ�ⳣ��Ϊ____________���������������䣬��8minʱѹ�������������1L����n(SO3)�ı仯����Ϊ_______������ĸ����
���±�Ϊ��ͬ�¶�(T)�·�Ӧ2SO2(g)+O2(g) ![]() 2SO3(g) ��H��0�Ļ�ѧƽ�ⳣ����K����
2SO3(g) ��H��0�Ļ�ѧƽ�ⳣ����K����
T/�� | T2 | T3 |
K | 20.5 | 4.68 |
�ɴ���֪������������ͬ����T1��T2��T3���ֲ�ͬ�¶��£���Ӧ�ӿ�ʼ���ﵽƽ��ʱ����Ҫ��ʱ������� _____________��������T1������T2������T3����
��2������ϵ��ѹ�������½��з�Ӧ��2SO2(g)+O2(g) ![]() 2SO3(g)��ԭ������SO2��O2�����ʵ���֮����k����ͬʱ��SO2��ƽ��ת�������¶���t���Ĺ�ϵ��ͼ2��ʾ��ͼ��k1��k2��k3�Ĵ�С˳��Ϊ____________��
2SO3(g)��ԭ������SO2��O2�����ʵ���֮����k����ͬʱ��SO2��ƽ��ת�������¶���t���Ĺ�ϵ��ͼ2��ʾ��ͼ��k1��k2��k3�Ĵ�С˳��Ϊ____________��

���𰸡� 66.7% 2.5 c T2 k1>k2>k3
��������(1)���ݻ�Ϊ2L�ܱ������г���0.6molSO3����ͼ1ƽ��ʱSO3���ʵ���Ϊ0.2mol��
2SO2(g)+O2(g) 2SO3(g)��
��ʼ��(mol)0 0 0.6
�仯��(mol) 0.4 0.2 0.4
ƽ����(mol) 0.4 0.2 0.2
��SO3��ת����Ϊ=![]() ��100%��66.7%��K=
��100%��66.7%��K=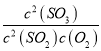 =
=![]() =2.5�������������䣬��8minʱѹ�����������0.5L��ƽ��������Ӧ�����ƶ���n(SO3)���Ϊͼ��c���ʴ�Ϊ��66.7%��2.5�� c��
=2.5�������������䣬��8minʱѹ�����������0.5L��ƽ��������Ӧ�����ƶ���n(SO3)���Ϊͼ��c���ʴ�Ϊ��66.7%��2.5�� c��
����Ϊ��ӦΪ���ȷ�Ӧ���¶�Խ�ͣ�ƽ��Խ������Ӧ�����ƶ���ƽ�ⳣ��Խ�������¶���͵�ΪK���ģ���T2���ʴ�Ϊ��T2��
(2)��ͬ�¶Ⱥ�ѹǿ�£�KԽС������Ũ��Խ��ƽ�������ƶ�������������ת����Խ����k1��k2��k3���ʴ�Ϊ��k1��k2��k3��






